Orthocell jumps again after dental membrane approved in USA
Health & Biotech
Orthocell (ASX:OCC) shares have soared to a 14-month high after the regenerative medicine company joined a short list of Australian medical devicemakers with a product approved for sale in the world’s largest economy.
Orthocell’s collagen membrane for dental and jaw surgeries has been approved for sale by the United States Food and Drug Administration, weeks after Australia’s Therapeutic Goods Administration gave it a similar tick.
Orthocell managing director Paul Anderson told Stockhead the “awesome” approval came sooner than expected but the company was already gearing up to meet expected demand.
“This is a good news story for Orthocell, it’s a good news story for the patients who are going to benefit from access for this technology, and it’s a really good news story for Australia,” he said.
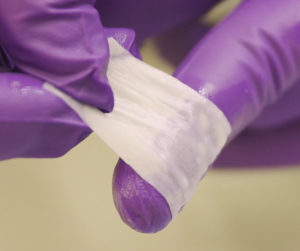
The CelGro Dentral membrane is being renamed Striate+ for the global launch and Orthocell has lined up endorsements from six key dental opinion leaders as it seeks to break into the $US500 million US dental market.
Orthcell says Striate+ offers superior handling and tissue integration than existing dental membranes on the market.
Approval of Striate+ also represents a checkmark for the company’s Perth manufacturing facilities, which should make it easier for the company’s other products to gain approval, Anderson said. Those products are cell therapies for regenerating damaged tendons and cartilage tissue.
Shortly before noon on Thursday, OCC shares were up 22.8 per cent to 56c, their best level since November 2019.