Genetic Signatures is heading to Europe to bust super bugs; shares gain 6pc
Health & Biotech
Health & Biotech
Digital superbug detector Genetic Signatures today announced it has the green light to sell its bug-busting kits to European hospitals.
The technology detects hospital “superbugs” or antibiotic resistant pathogens which Genetic says are a significant global concern in healthcare settings.
In Australian hospitals, 165,000 patients contract infections each year, university researchers found in a 2017 study.
Genetic’s detector kit gathers results in fewer than three hours – with minimal hands-on time for lab technicians – meaning doctors can dispense solutions faster.
The World Health Organisation warned of the prevalence of superbugs earlier this year.
It said new antibiotics need to be developed urgently to combat 12 families of bacteria, describing them as ‘priority pathogens’ and the greatest threat to human health.
While cures might be in the works, early identification can help to nip the outbreak in the bud.
“We are very excited to receive a major international approval for our EasyScreenTM technology,” chief Dr John Melki told investors.
“This registration follows significant work from the Genetic Signatures team and ultimately reflects the significant potential of our 3baseTM technology to make a real difference in pathogen detection and treatment processes.”
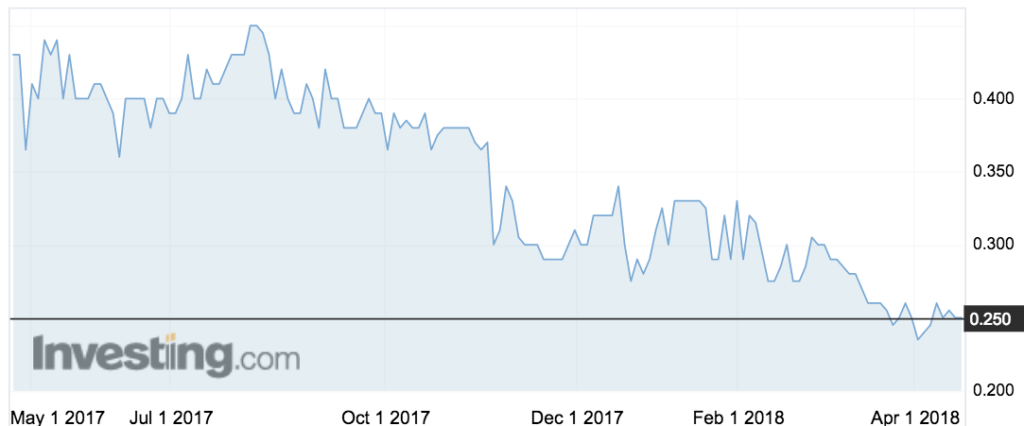
Shares in the company were trading steady at 25c in morning trade – near the bottom of its 52-week range, from heights of 45c last August.