Health & Biotech
Atomo Diagnostics (ASX:AT1) is an Australian manufacturer of medical devices used in blood-based rapid testing. During the COVID-19 pandemic and associated volatile markets, Atomo Diagnostics was the first company to list on the ASX after a six week period of inactivity. This came after a successful capital raising effort adding $30 million.
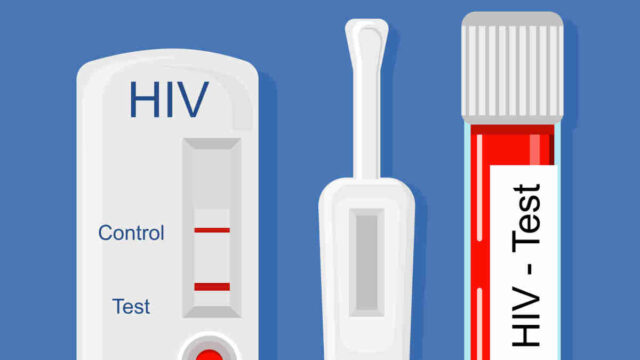
Since its founding in 2010, the company’s specialty has been in HIV testing. Its solutions, which include a self-test kit and professional-use kit, are approved in Australia and Europe.
Since 2015 it has sold 550,000 rapid diagnostic tests (RDTs) direct to medical professionals and consumers as well as another 430,000 to other RDT manufacturers for sub-assembly. These work similar to a pregnancy test — a liquid sample, such as blood is applied to the test strip and if the target substance is present, two lines will appear.
More recently, Atomo Diagnostics has been working on self-testing kits for COVID-19. The company says that it can deliver results in as little as 15 minutes. Atomo has reported substantial demand for the test, which screens the blood for antibodies generated in response to the virus. The company has set aside an initial 300,000 units in inventory to be made available for COVID-19.
Atomo’s revenues to date pertain mainly to sales of the HIV test in Europe, Africa, Central and South America and southeast Asia. Atomo Diagnostics has ‘pre-qualified’ status with the World Health Organisation for the HIV self-tests, which in effect qualifies the company as a vendor to the health body’s third world health programs.
In April 2020, Atomo Diagnostics made its ASX debut, rocketing to 45c — a 125 per cent gain on its IPO price. During the pandemic, Atomo made 300,000 RDTs available to specifically test for COVID-19. By the end of the month, the company had more than doubled its share price since listing.
In August, the Therapeutics Goods Administration approved Atomo Diagnostics’ AtomoRapid COVID-19 antibody test for use in the country. Atomo is working with Partners to bring it to market. Meanwhile, Atomo Diagnostics has supplied millions of its rapid, self-administered AtomoRapid tests to French biotechnology specialist NG Biotech and US company Access Bio for distribution in France and the United States.
RELATED STOCKHEAD STORIES
Health & Biotech
Health Check: Chinese consumer market is a thing of beauty, says EZZ Life Sciences
Health & Biotech
Health Check: Painchek strives to Make American Aged Folk Comfortable Again
Health & Biotech
Health Check: Which biotech boards are at risk of a ‘second strike’?
Health & Biotech
Health Check: Despite headwinds, Australia’s third force in pharmacy finds prescription for growth
News
Rise and Shine: Everything you need to know before the ASX opens
News
Closing Bell: Investors wag their tails as ASX rallies hard; real estate, goldies, copper stocks all gain
News
ASX Small Caps Lunch Wrap: Who’s regretting going DIY with a poop transplant this week?
Health & Biotech
ASX Health Stocks: Atomo up over 20pc on Budget boost for HIV self-test kits
News
Top 10 at 10: HIV budget boosts and a couple of promising copper, gold drill campaigns
Health & Biotech
ASX Health Stocks: Atomo sells more HIV Self-Tests; Actinogen begins Phase 2b trial on Alzheimer’s
News
ASX Small Caps and IPO Weekly Wrap: Gold and medicine are the best medicine
News
Rise and Shine: Everything you need to know before the ASX opens
News
Closing Bell: Cettire rejects AFR claims, GDP’s gone back to the 90’s and ASX200 ends above the ice
News
ASX Small Caps Lunch Wrap: GDP drama is over, now markets brace for the lack of drama, drama
Health & Biotech
ASX Health Stocks: Atomo pops 90pc after purchase order; AdAlta up 20pc on Phase 1 results
News
Top 10 at 11: Dicker Data founder sells $90.47 million worth of stock following divorce settlement
Health & Biotech