Why this ASX biotech is primed to capitalise as big pharma flocks to the rare diseases market
Health & Biotech
Health & Biotech
Special Report: Newly appointed CEO of clinical-stage biopharmaceutical development company PharmAust Dr Michael Thurn says the company is well-positioned to capitalise on the lucrative rare diseases market.
Thurn started as CEO on September 1 with more than 25 years in the biotechnology industry including a stint with TGA regulating drugs and private companies spun out of universities, particularly the University of Queensland.
He was founding managing director of Spinifex Pharmaceuticals, noting similarities between its lead program in pain management and where PharmAust’s (ASX:PAA) is with its motor neurone disease/amyotrophic lateral sclerosis (MND/ALS) program.
“That particularly program at Spinifex was sold for around US$700 million to Novatis,” Thurn says.
PAA’s main business model is repurposing veterinary product monepantel, used to treat parasitic infections in sheep, for the treatment of canine cancers and human neurogenerative diseases.
Thurn says the advantage in repurposing monepantel is PAA can leverage data from its canine program into its human programs and vice versa.
“We can make the advancements of both those programs into a position of being commercialised more straight-forward and cost-effective,” he says.
“We can use data from both programs and existing data which has been built up over time from the approved veterinary product.”
PAA announced in October completion of its Phase 2 veterinary clinical study of monepantel treatment for canine B-Cell lymphoma.
PAA will now file an investigational new animal drug with the US FDA, paving the way to advance into a field study in the US early next year in about 60 dogs, lasting for about 12-months.
“The field study will position the program for near term commercialisation and the ability to gain conditional approval from the FDA,” Thurn says.
Studies are showing no severe adverse reactions, no treatment-related deaths and point towards safe efficacy of the drug to treat canine B-Cell lymphoma, which has a poor prognosis.
“We found we had a very similar efficacy profile to the latest drug approved for B-Cell and T-Cell lymphoma LAVERDIA,” Thurn says.
“The interesting thing about LAVERDIA is that the US sales rights were sold to one of the main US veterinary pharmaceutical companies Dechra for around US$64 million.”
He says a key difference of monepantel and other treatments available for B-Cell lymphoma is the high quality of life and function they observed during the trial.
“Generally, the vet says there is chemotherapy or there is euthanasia, and most people decline chemotherapy because of the considerable costs involved and safety considerations for both the animal and its owners,” he says.
“We hope to provide a new treatment paradigm around a high-quality of life for an animal at home so you can still go out and play catch.
“It’s a therapeutic product so there are no issues in regard to having to wear gloves, quarantine the animal and they will appear normal because of the high-quality of life and function so their normal daily routine continues.”
Thurn says PAA is now at a pivotal point with its Phase 1 MND/ALS trial, announcing in October it had completed dosing in the trial and is on track to release results in Q1 CY24.
The Phase 1 MND trial is fully funded by the Australian Fight MND campaign. Thurn says there is no cure for MND/ALS with life expectancy on average after diagnosis 27.5 months.
“There’s predictions that by 2040 incidences of MND/ALS will increase 70% largely due to environmental factors causing a rapid prevalence of the disease,” he says.
Thurn says there is only four drugs approved for the disease currently, with few in the pipeline.
“The real blueprint for us was Relyvrio which was approved in September 2022 and that prolongs life for nine months,” he says.
“The important aspect about Relyvrio is that it was approved on Phase 2 data in just under 100 patients.”
Thurn says there is a good prospect once monepantel moves into a Phase 2 trial for MND/ALS to potentially receive accelerated approval.
He says in MND/ALS there is a progressive degeneration of nerve cells, caused by a build-up of toxic protein and metabolic waste over time.
Furthermore, he says that process is inherent in the degeneration of nerve cells across most neurodegenerative diseases, potentially making it an effective treatment beyond MND/ALS.
“Monepantel promotes and enhances autophagy which is the natural process the cell uses to clean up toxic protein build up, metabolic waste and allows the recycling of nutrients,” he says.
Thurn says most of the patients who entered PAA’s Phase 1 study had been diagnosed with the disease for 24 months.
“A lot of patients have now been on the drug for 13 months, which is an incredible result when you consider the life expectancy of 27.5 months from diagnosis,” he says.
Thurn says progression of its programs show it is now a strong contender to compete in the rare diseases market, which reached more than US$195 billion in 2022 and is forecast to reach US$435 billion by 2032 with a CAGR of >8.5%.
He says the number of deals done in the rare disease market is increasing as big pharma realise the potential of an orphan drug designation (ODD).
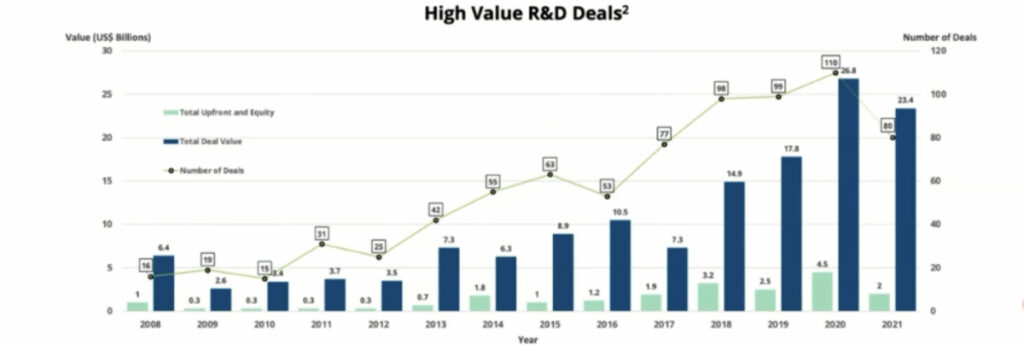
“What you can see is from 2018 onwards the average number of deals done in rare diseases is about 100 as big pharma recognise the advantages of an orphan disease designation,” Thurn says.
He says these include increased access to the FDA, new drug application fee waivers, a potentially faster route to market and additional seven years of exclusivity once the drug is approved.
“We’ve submitted an orphan drug designation application with the FDA and expect to hear back in January next year and that enables us to start to think about being a data point on this particular graph,” Thurn says.
“Big Pharma are flocking to rare diseases market because of financial and regulatory incentives, along with getting access to a larger market.
Thurn says generally that orphan disease has applicability in other mainstream applications.
“In PharmAust’s case big Pharma could see getting orphan drug designation to getting quick approval through the FDA for motor neurone disease,” he says.
“They could then potentially off-label prescribe into a greater market like Alzheimer’s, Huntington’s, Parkinson’s and the various other more prevalent neurodegenerative diseases.”
This article was developed in collaboration with PharmAust a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.