ResApp won’t be diagnosing croup with its coughing smartphone app

ResApp Health (ASX:RAP), the maker of a smartphone app designed to help clinicians diagnose respiratory disease in children, won’t add croup to its diagnostic lineup after it was unable to gather enough evidence to support its efficacy.
ResApp told investors this morning that the long-awaited results for croup, an upper airway infection that blocks breathing and has a distinctive barking cough, were positive, but not strong enough to submit a regulatory filing in the US.
The company is preparing to file a submission to the US Food and Drug Administration (FDA) to have its app approved for diagnosing lower respiratory tract disease, asthma and reactive airway disease and primary upper respiratory tract disease.
It was hoping to add croup to that list of diseases but the low incidence of croup in the patient population meant there was not enough data to support it — only 2 per cent of the study population of 29 patients were clinically diagnosed as having croup. A larger population would be required to support a US regulatory filing for croup, the company said.
Shareholders were cautiously optimistic, sending shares up 2 per cent to 9c.
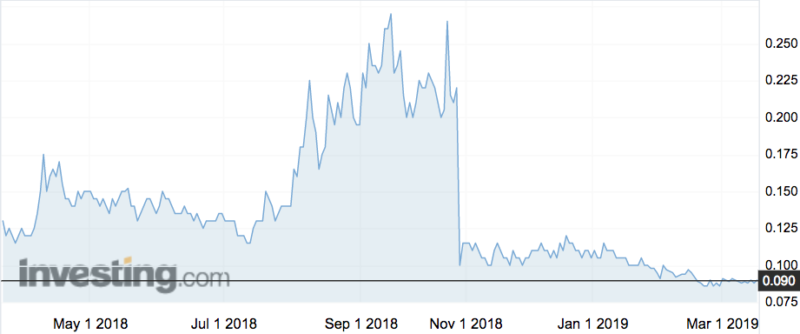
ResApp has taken shareholders on a rollercoaster ride over the past few years with its coughing app. Its share price collapsed in 2017 when an earlier trial failed and had to be redone.
In October last year it reported mixed results from that trial, which as the chart above shows, caused another significant fall in the share price.
Data for lower respiratory tract disease, asthma and reactive airway disease and primary upper respiratory tract disease was strong enough to support a submission to the FDA, but pneumonia results were poor and figures for croup were delayed “because of a technical issue in providing cough audio files to adjudicators for review”.
Boss Tony Keating later admitted his company could have done a better job of educating its shareholders on what a good result looked like.
He told investors today ResApp was on track to get its app approved.
“We are very satisfied with the croup diagnostic results that we achieved, especially when compared to the treating team’s accuracy, though low incidence of croup in the study population demonstrates the relative rarity of croup in this setting and prevents us from including it in our FDA submission,” he said.
“We expect to file a De Novo submission to the US FDA this month for three of the most clinically-important indications: lower respiratory tract disease, asthma/reactive airways disease and primary URTD.
“These three indications provide valuable information to clinicians at key decision points and are relevant to every single patient who presents with signs or symptoms of respiratory disease.”
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








