ResApp boss: shareholders not educated enough to recognise good results

Pic: Oscar Wong / Moment via Getty Images
ResApp boss Tony Keating has assured investors the biotech is on the right path despite this week’s share price tumble following long-awaited trial results from its coughing app.
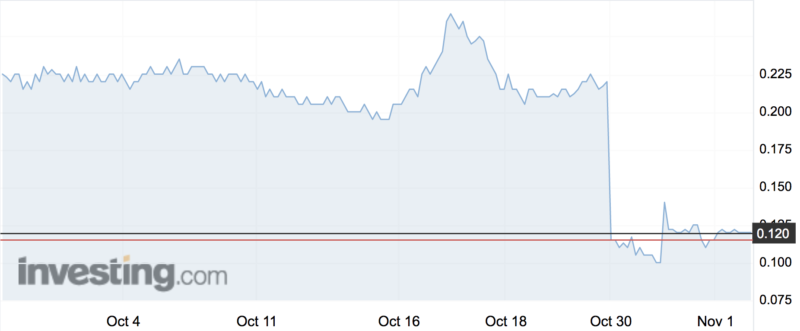
ResApp’s share price (ASX:RAP) more than halved on Tuesday after the company reported “positive preliminary results” for its SMARTCOUGH trial that some shareholders called into question.
ResApp is developing a smart phone app that’s designed to diagnose respiratory disease.
Dr Keating told Stockhead at the TechKnow Invest Roadshow conference in Melbourne that ResApp had “perhaps not communicated well enough” in preparing investors for the news.
“The results from the US are really positive, but I think we can understand that we haven’t educated shareholders well enough on what a good result actually looks like,” he said shortly after presenting at the Melbourne leg of the conference on Thursday.
“We can understand that the shareholders didn’t quite have the information well enough to make that clear.
“We probably needed to bring to the forefront how complex the process is to have these clinical diagnoses assessed.”
ResApp spent nearly a week in a trading halt as it put the finishing touches on the initial results — twice delaying an announcement before finally releasing on Tuesday morning.
The share price fell from 22c to an intraday low of 9c, a drop of 59 per cent. It closed the day at 10c, though has recovered today to a high of 12.5c.
A long history
The trial has a long history. Fourteen months ago the company’s share price collapsed 77 per cent when an initial trial failed.
Its shares began to slowly recover after announcing it would re-run the trial from January this year.
Dr Keating said the outstanding results they received in the Australian version of the trial had “exceeded our expectations”, which may have contributed to a misunderstanding this week of the US results.
“There are differences between the US and Australia that naturally affected the numbers,” he said.
“There are many differences in clinical practices, the US healthcare system is very very different, it’s very privately funded, focused on reimbursement and there is a lot of testing because of the malpractice problems that the country has.
“There are twice as many chest x-rays for pneumonia in the States, for example, than Australia, so that is going to affect the practice of medicine.
“And so pneumonia proved to be a challenge for us in this trial with lower numbers than in Australia. But for us the other indications are more exciting commercially and a big opportunity for us.”
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
ResApp told investors the latest trial results would lead to regulatory submissions.
“These results have opened up a new stage of the company,” he said. “We now move into regulatory submissions, hopefully approvals and then it’s on to commercialisation.
“We’ve done things really quickly, taking just three years to go from a university idea to making regulatory submissions, the equivalent of a successful Phase 3 biotech — that just doesn’t happen in this sector.”
Dr Keating said the secret to the company’s resilience after a number of setbacks was the strength of its team.
“Following the last failure where we had bad execution problems, no one left the company,” he said. “Everyone stayed on board and worked really hard to get us to the point we are at today.
“So working with a great team, with great clinicians and a lot of great, patient shareholders. That’s the gratifying part for me.”
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








