What ResApp’s boss will do next after his 77pc share price collapse

Pic: Charnchai / iStock / Getty Images Plus via Getty Images
Tony Keating says the poor trial results that destroyed his company’s share price were because he underestimated the challenge of testing inside a hospital emergency department.
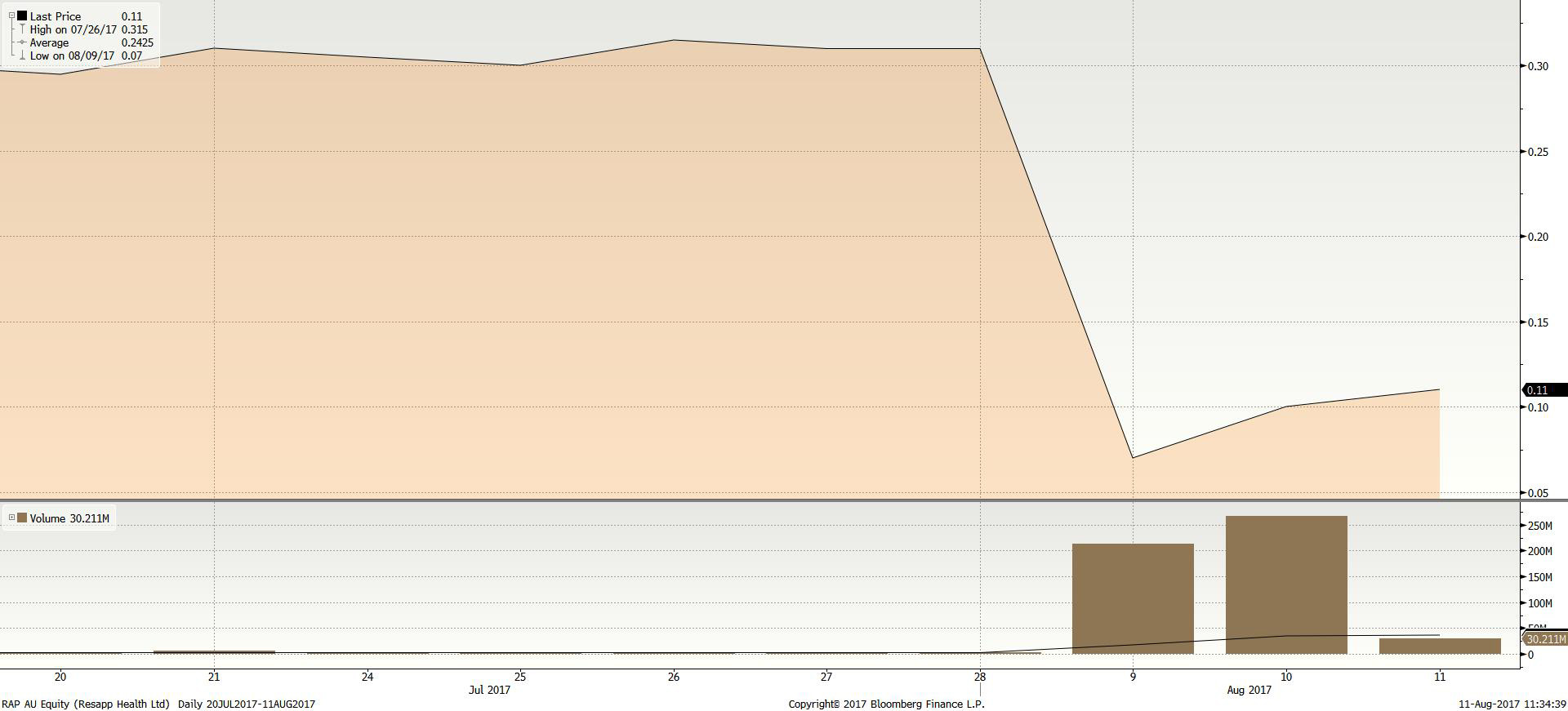
Mr Keating is the boss of health tech company ResApp (ASX:RAP). ResApp’s price fell almost 80 per cent on Wednesday after disappointing trial results for its app designed to diagnose respiratory disease.
About 40 per cent of the company’s shares on issue changed hands in 48 hours. The shares are currently sitting at about 10c.
“I’m very disappointed for my shareholders, who have obviously taken a big hit from this,” Mr Keating said in an interview with Stockhead.
“My goal right now is to keep our shareholders and clinical partners excited about moving forward. That’s really my goal here, to refine the path that we’ve laid out in front of us and to get everyone on the same page.”
That path forward is to do the trial again, and do it better — and Mr Keating says ResApp has the cash to make it happen.
“We don’t really think the results here are representative of the true performance of the app,” he said of the SMARTCOUGH-C trial, which was conducted on children 12 years and under at the Massachusetts General Hospital, Cleveland Clinic and the Texas Children’s Hospital in the US.
“The way this trial was run, and this is an issue for all of us, is that the algorithms were not tested in a fair manner.”
So what went wrong?
Mr Keating said the problems came from running the trial in the high-pressure environment of an emergency department, and with children.
“We misjudged the complexities of the US emergency department system and the challenges of running a clinical trial in a US emergency department,” he said.
“Our clinicians are now working with us so they get a better idea of what we require, and we understand the challenges of working within an emergency department.”
Some of those challenges, as outlined to the market earlier this week, involved poor quality of cough recordings and the fact many patients were treated prior to using the app.
Mr Keating said the results of previous testing in Australia, on adults, showed that the app can work.
“We’ve run an Australian program in adults for the last 18 months and it had great results from that program,” he said.
The plan for ResApp now is to look closely at what went wrong with the SMARTCOUGH-C trial and launch another US trial over the summer.
“We’ve only had these results for 60 hours,” Mr Keating said.
“Right now we’re spending some time going on a case-by-case basis through the data that we have — that’s reviewing the data for the recordings, reviewing the clinical data, reviewing the algorithm performance.
“That work will probably take until the end of August, at which time we will make an announcement to the market about the findings.
“We will then initiate another study in the US winter, around about November.”
‘You can call my mobile’
Mr Keating held a phone conference for investors on Thursday, with about 250 people dialling in.
“I’m very happy we did that call, it gave me a chance to speak to everybody and give them more colour and context around the study and outline the path forward,” he said.
“It was very well attended. The shareholders were very reasonable and I fielded a lot of intelligent questions.
“We have always made ourselves very transparent to investors. My mobile number is on every press release, that’s one of the things I think we’ve done very well in the past and we will continue to do.”
ResApp spent $1.82 million in the quarter to June, leaving $8.56 million in the bank. It expects to spend another $2.29 million in the current quarter.
The algorithms used by ResApp were originally developed by the University of Queensland with funding from the Bill and Melinda Gates Foundation.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








