PharmAust’s MPL could offer MND/ALS patients up to 56.5 months additional life expectancy
Health & Biotech
Health & Biotech
Special Report: PharmAust’s drug monepantel may be the key to delivering significant improvements in life expectancy for sufferers of MND/ALS with preliminary Phase 1 data showing a 58% reduction in disease progression.
MND/ALS (motor neurone disease/Amyotrophic Lateral Sclerosis) – an invariably fatal nervous system disease that weakens muscles and impacts physical function – affects over 350,000 people globally.
Sufferers have an average life expectancy of about 27 months with FDA approved treatments Riluzole and Relyvrio only able to prolong life by about 2-3 months and 10 months respectively.
PharmAust (ASX:PAA) first identified the potential of monepantel (MPL) to treat MND/ALS after pre-clinical studies found the drug could activate molecular pathways relevant to its development as a MND therapy.
This led the company to launch the Phase 1 MEND study for MPL in patients with MND/ALS that was successfully completed late last year.
Phase 1 trials are aimed primarily at establishing the safety, best dosage and timing of the new treatment.
No deaths or significant adverse events were recorded during the trial and all 12 patients, who remain able to swallow and breathe unassisted, elected to continue treatment with MPL through a compassionate-use program that will also serve as a 12-month open-label extension (OLE) study that will explore the prolonged safety, tolerability and effectiveness of MPL.
Adding further interest for the company, the Phase 1 study also returned some early indicators into the efficacy of MPL with only one of the 12 patients showing an increase in Neurofilament Light Chain (Nfl) protein concentrations in their plasma.
Research has suggested a correlation between Nfl increases and MND progression.
PAA has now released top-line results for the Phase 1 MEND study which shows MPL has met its primary safety and tolerability endpoints, which are superior to those demonstrated by Relyvrio.
The study found no dose-limiting toxicities were experienced and that of the 56 treatment‑emergent adverse events (AEs) reported, only three AEs – which were graded mild – were considered to be possibly related to the treatment.
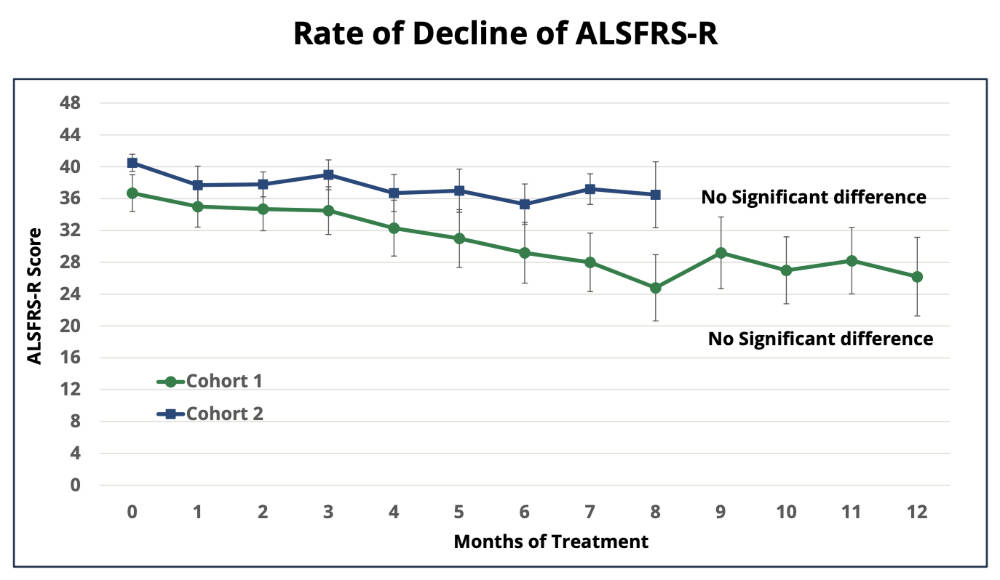
Adding further interest for PAA, the results also demonstrate a positive signal of potential efficacy with preliminary data highlighting a 58% reduction in the rate of disease progression for Cohort 2 (High Dose) using the FDA primary efficacy endpoint, ALS Functional Rating Scale-Revised (ALSFRS-R).
ALSFRS-R assesses for changes in a person’s physical abilities over time of 12 specific functions including speech, walking, climbing stairs, dressing/hygiene, handwriting, turning in bed, cutting food, salivation, swallowing and breathing.
The study found no significant differences in ALSFRS-R scores between pre-dose and end of treatment for all 12 patients, an indicator that treatment with MPL had slowed the rate of disease progression.
Separately, concentrations of MPL sulfone (MPLS) – the active metabolite of MPL – increased proportionally with the higher doses of MPL.
MPLS was found in the cerebrospinal fluid indicating that both MPL and MPLS have the ability to cross the blood brain barrier.
Using a prognostic predictor model – and depending on the current severity of the disease – a 0.48 slope change, as calculated for all 12 patients in the study, could provide patients with an additional 13.5-56.5 months in median survival. Currently, approved treatments for ALS/MND provide approximately 2-9 months in additional life expectancy.
Study principal investigator associate professor Dr Susan Mathers said the study has shown oral MPL to be safe and well tolerated by people with MND.
“Given the promising findings on preliminary efficacy markers, I look forward to progressing a Phase 2 study as soon as possible,” she said.
PAA chief executive Dr Michael Thurn said the release of the top line results represented an exciting milestone for the company as it took a significant step towards helping people diagnosed with MND/ALS.
“The 58% slowing in ALSFRS-R decline amongst Cohort 2 participants clearly demonstrates the potential to provide meaningful clinical benefit to people living with MND/ALS,” he said.
“To know that we have potentially prolonged the lives of 12 patients is extremely satisfying and humbling. We now look forward to advancing discussions with strategic partners who share our vision for monepantel.”
PharmAust recently unveiled significant findings from a statistical review indicating a notable survival benefit.
The review, undertaken by statistical experts partner Berry Consultants, found if 12 similar MND patients went untreated, the likelihood of any surviving is less than 0.1%, or 1 in 1000.
To assess the likelihood of a 100% survival rate among the 12 Phase 1 MEND patients without treatment, the statistical experts used baseline survival rates derived from historical control data within the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database.
The PRO-ACT database is the largest publicly available repository of merged ALS clinical study data.
PAA has identified an optimal dose of MPL for the pivotal Phase 2/3 clinical study, which is expected to start in Q3 2024.
Success is potentially very lucrative for the company as the total MND/ALS addressable market is US$3.6 billion per annum with Riluzole having already achieved annual peak sales of ~US$1 billion.
This article was developed in collaboration with PharmAust, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.