Paradigm jumps 20pc on positive Phase 2 trial osteoarthritis results, including ‘notable increase’ in cartilage thickness
Health & Biotech
Health & Biotech
Paradigm Biopharmaceuticals has surged after unveiling positive new analysis from its phase 2 clinical trial of injectable pentosan polysulfate sodium (iPPS) for treatment of osteoarthritis (OA) in the knee.
Paradigm Biopharmaceuticals (ASX:PAR) says a single 6-week treatment course of (iPPS) showed pain reduction and functional improvement effects through the 12-month study with notable increase of cartilage thickness at 6 months.
The phase 2 clinical trial was performed concurrently with Paradigm’s ongoing phase 3 study.
Paradigm says this is a first — no OA drug has previously shown durable and meaningful improvements in pain and function at 12 months after a single course of treatment.
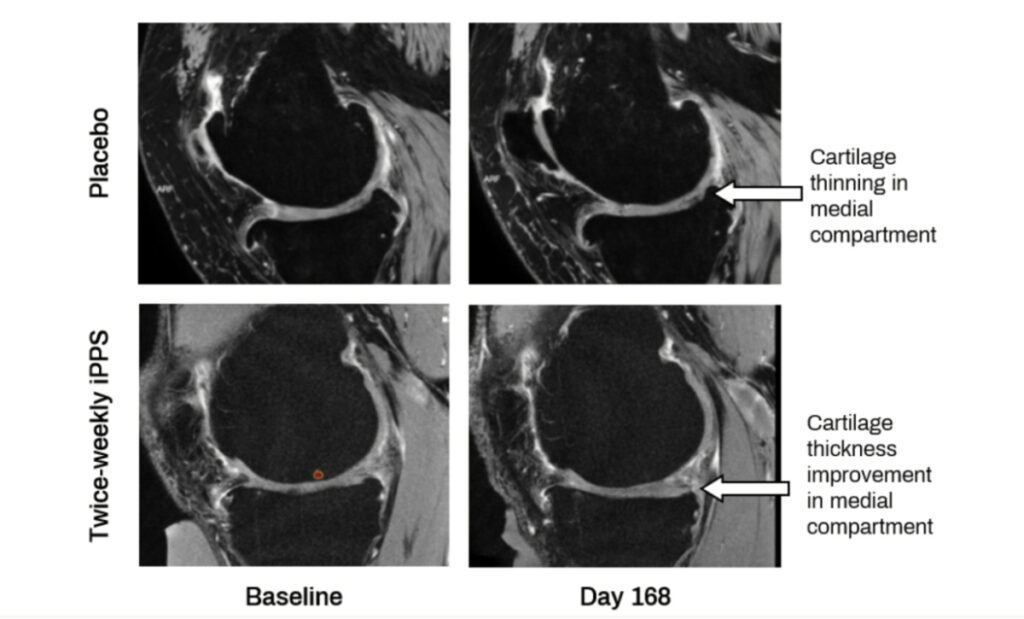
The company previously reported attaining the primary endpoint at Day 56, significant improvement in pain and function at Day 56, and changes to molecular and structural biomarkers of OA disease progression at Day 168. The MRI results at Day 168 have now undergone extensive quantitative analysis.
In the Phase 2 clinical trial, participants receiving 2 mg/kg iPPS twice weekly for 6 weeks, reported objective changes in structural biomarkers of knee OA by MRI at 6 months (Day 168) compared to placebo as follows:
In short, these results establish that a short course of iPPS showed positive structural changes in the affected knee joint and offered clear signs of how OA can be effectively treated.
iPPS has been shown to both treat the symptoms of OA and to preserve and/or regenerate joint tissues.
Paradigm says this is significant from a commercial perspective because the disease modifying effects of iPPS observed in the phase 2 clinical trial are expected to support a greater reimbursement compared to that which would be expected for a therapeutic that would only treat the symptoms of OA.
PAR says cartilage loss is generally considered to be the most important structural disease feature of OA.
Cartilage serves as a cushion and provides smooth, low-friction movement in the knee joint.
When cartilage degenerates, it can lead to bone-on-bone contact, resulting in severe pain.
Numerous studies have shown cartilage loss to be predictive of knee replacement surgery for OA.
There has been substantial published research on MRI cartilage measurements showing that as OA progresses, the medial side of the knee tends to lose cartilage more rapidly than the lateral side.
A reduction of loss, or an increase in cartilage thickness, may therefore delay time to total knee replacement (TKR) in an osteoarthritic knee.
PAR says any improvement in cartilage health can help alleviate pain, improve joint functionality, and improve the quality of life for people with knee osteoarthritis.
The most common form of arthritis is OA, frequently known as degenerative joint disease or wear and tear arthritis.
OA primarily appears in the hands, hips, knees, and feet and involves the progressive breakdown of the protective cartilage within a joint, resulting in changes to the underlying bone.
In 2022, the global market for osteoarthritis therapeutics was valued at $8.28 billion, and it is anticipated to reach approximately $20.24 billion by 2032.
Paradigm says it has identified the optimal dose for achieving durable responses in pain, function, and patient global impression of change (PGIC).
The 2 mg/kg dose administered twice weekly was not initially included in Stage 1 dosing of the phase 3 study.
Consequently, the company requested the Data Monitoring Committee (DMC) perform an interim analysis of the treatment outcomes across all dosage groups during stage 1 of the phase 3 trial.
The analysis showed that all the stage 1 doses (less than 2 mg/kg twice weekly) for the phase 3 trial are not as effective.
As a result, Paradigm has decided to incorporate the 2 mg/kg twice weekly regimen into their OA development program.
The company says it is progressing with the TGA Provisional Approval application which would expedite the pathway to marketing approval in Australia.
It is also moving forward with the 2 mg/kg twice weekly optimal iPPS dose for registration programs for the improvement of pain and function in knee OA.
The company says the data is expected to further support partnering discussions.
Managing director Paul Rennie says the treatment of OA has not progressed for many years, with available therapies mostly treating the symptoms of the disease for a short period.
“There is a high unmet need for a new OA therapy to slow OA progression in tandem with symptomatic improvement of pain reduction and functional improvement,” he says.
“We look forward to presenting the (Phase 2) data package to global regulatory agencies including the TGA and FDA.
“What I find most compelling about the data, is that all participants entered the clinical study with active structural degeneration already occurring and yet iPPS has been able to reverse disease progression.
He says Paradigm aims to reach agreement with the US FDA on what data would be necessary to confirm these results in its Phase 3 program to include disease modifying data on its label at registration.
This article was developed in collaboration with Paradigm Biopharmaceuticals, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.