- Neurotech Phase 2/3 NTIASD trial in autism spectrum disorder meets primary endpoint
- Study showed a statistically significant improvement in severity of illness in paediatric patients treated with NTI164
- Neurotech undertakes $10 million placement to further advance clinical trials
Special Report: Neurotech International has reported positive outcomes from its Phase 2/3 NTIASD2 clinical trial targeting children diagnosed with Autism Spectrum Disorder (ASD).
Clinical-stage biotech Neurotech International (ASX:NTI) says its Phase 2/3 NTIASD2 trial successfully met its primary endpoint and key secondary endpoints with comprehensive data analysis and interpretation underway.
Enrolling a cohort of 54 patients categorised at Level 2 (requiring substantial support) and Level 3 (requiring very substantial support) autism, all NTIASD2 participants were recruited through the Paediatric Neurology Unit at Monash Medical Centre under the guidance of principal investigator Professor Michael Fahey.
NTI164 is a proprietary drug formulation derived from a cannabis strain with low THC (M<0.3%) and a novel combination of cannabinoids including CBDA, CBC, CBDP, CBDB and CBN.
The drug has been exclusively licenced for neurological applications globally with pre-clinical studies demonstrating a potent anti-proliferative, anti-oxidative, anti-inflammatory and neuro-protective effects in human neuronal and microglial cells.
NTI is developing NTI164 for a range of neurological disorders in children where neuroinflammation is involved.
In further good news for NTI, the company reporting positive initial findings today of its Phase 1/2 clinical trial (NTIRTT1), showcasing the efficacy and safety outcomes of NTI164 in treating Rett Syndrome.
The randomised, double-blind, placebo-controlled clinical Phase 2/3 NTIASD into ASD aimed to determine the efficacy and safety of NTI164 versus placebo.
The study comprised an eight-week treatment period followed by an eight-week open-label maintenance period followed by a two-week wash-out period. There were 26 patients in the NTI164 arm and 28 patients in the placebo arm.
The average age was 12.4 for the NTI164 arm and 12 for the placebo arm with 54% in both arms’ male and 46% female.
Throughout the 8-week randomisation period, no severe adverse events occurred in either the NTI164 arm or the placebo arm.
During this timeframe, a combined total of 11 adverse events were documented across 7 patients in both arms.
No changes to kidney/liver function over the 8-week trial period were noted with treatment-related diarrhoea and nausea/vomiting rate lower for the NTI164 arm.
None of these events were deemed severe, nor did they significantly impede the patients’ functionality.
Meaningful improvement for treatment
NTI says Clinical Global Impression –Improvement (CGI-I) is a seven−point scale reflecting clinical judgment of the patient based on the clinician’s total experience with the ASD population graded from one (very much improved) to seven (very much worse).
A decrease in CGI-I score indicates improvement. There was a strong treatment effect/benefit observed of -1.42 (95% CI; -2.0, -0.82) in the NTI164 group versus placebo at eight weeks, which the company says was highly statistically significant (p<0.001).
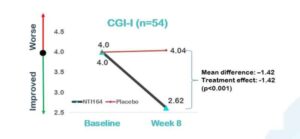
Source: NTI
Professor Michael Fahey, head of paediatric neurology at Monash Children’s Hospital, says the NTIASD2 trial looks promising with a substantial unmet market need for safe and effective therapies for autism, like NTI164.
“The analysis so far of the trial, which compared NTI164 to placebo over eight weeks of daily treatment, have demonstrated statistically significant and clinically meaningful improvements in the severity of illness and adaptive behaviours such as communication and socialisation without any significant side effects,” he said.
“Currently, there are no FDA or TGA-approved treatments that show clinically significant improvements in one or more of autism’s three core symptom domains – communication, impaired social interaction, and restricted behaviours.”
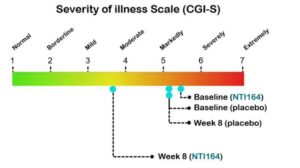
Source:NTI
NTI executive director Dr Thomas Duthy says the trial results “absolutely and unequivocally confirm earlier clinical findings for NTI164 in ASD”, further demonstrating substantial clinical benefits in children with ASD cross multiple measures.
“We are exceptionally thankful to Professor Fahey and his clinical team, patients, and their caregivers for their participation in this important clinical trial that has provided strong evidence of improvement in ASD patients in a well-controlled manner,” he said.
Duthy noted the NDIS’s substantial spending on participants with Autism highlights a dire need for a safe and effective therapeutic intervention to improve ASD symptoms and reduce healthcare costs.
“The results enable NTI to aggressively explore its commercialisation options in light of the significant market need and patient/caregiver pull for new safe and effective interventions to improve these children’s lives,” he said.
Duthy says patients previously assigned to the placebo arm are now eligible for an additional eight weeks of NTI164 treatment, with the option for all patients to extend treatment for up to 52 weeks, should they choose to do so.
NTI plans to expedite discussions with regulatory authorities, particularly with the Therapeutic Goods Administration in Australia, due to the strength of the trial data.
$10 million capital raise to advance trials
NTI also announced today a $10m placement for the issue of 100m new fully paid ordinary shares to institutional, professional, and sophisticated Australian and overseas investors.
The placement’s issue price is 10c a share, offering a 4.8% discount to the last closing price of 10.5c before the trading halt was called to prepare for the clinical results and capital raise.
NTI says the price also reflects a 3.5% discount to the 5-day VWAP of 10.36c and a 4.6% discount to the 15-day VWAP of 10.49c.
Placement participants will receive one free attaching option for every two New Shares subscribed, totalling 50 million new options.
The options will have a two-year expiry from the date of issuance and a strike price of 16c. NTI plans to issue the new shares around April 24, 2024.
NTI says placement proceeds will go towards clinical trials as needed, regulatory development efforts, toxicology initiatives for IND enabling, product manufacturing and expansion, expenses related to the placement, and general working capital needs.
Duthy says the company and board thanks investors for their strong support of the placement, and welcomes new institutional investors, following the delivery of clinical results in ASD along with top-line Rett Syndrome data, which met the primary endpoint.
“Our corporate strategy has expressly focussed on NTI164 clinical development in paediatric neurological disorders with persistent or progressive neuroinflammation,” he says.
“This has resulted in all our clinical trials launched and recruited over the last 12 months delivering statistically significant and clinically meaningful results in three very challenging neurological disorders where effective treatments are lacking.
“These include ASD, Rett Syndrome and PANDAS/PANS with the latter two disorder classified as rare or orphan diseases.”
This article was developed in collaboration with Neurotech International, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
You might be interested in














