Cancer sniper Prima BioMed rebrands to Immutep as it zeroes in on ‘immuno-oncology’
Health & Biotech
Health & Biotech
Cancer drug developer Prima BioMed has cemented its focus on immunotherapy, changing its name to Immutep as it heads into further trials for treatment of advanced breast and skin cancer.
Named for the Egyptian god of medicine (not the villain in the Brendan Fraser movie) — and a French biotech acquisition made in 2014 — Prima (ASX:PRR) will be known as Immutep (ASX:IMM) from December 1.
It is a deliberate step by the company to distance itself from its divested ovarian cancer vaccine that was sold off several years ago.
Now, all the focus is on a protein called lymphocyte activation gene 3 (LAG-3), that can identify cancer cells to regulate immune responses, allowing patients to fight the disease using their own cells.
The company is in late-stage trials for the treatment of advanced breast cancer in Europe and will progress to Phase II trials for advanced Melanoma in Australia next year.
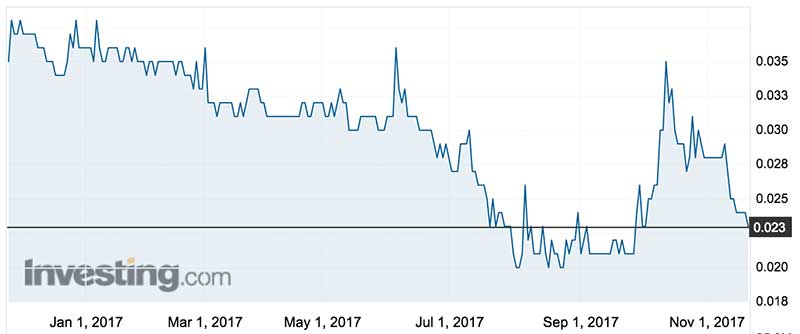
Prima BioMed/Immutep shares were trading down 4 per cent at 2.3c on Tuesday, valuing the company at $54 million.
Chief executive Marc Voigt told Stockhead the latest clinical trials were promising, and showed signs of potential where other immunotherapy treatments did not.
“Immuno-oncology will be the backbone of oncology in the next five to ten years — this is not a trend,” Mr Voigt said. “It is underpinned by data and by products already on the market like Keytruda.”
In simple terms, existing therapies such as Keytruda act as a brake on cancer cells, while Prima’s LAG-3 protein acts as an accelerator to enhance production of immune cells that fight them.
Early findings from a melanoma study were released earlier this month, showing the company’s LAG-3 protein demonstrated anti-tumour activity when paired with Keytruda — even when Keytruda alone had not been beneficial.
Cancer Council chief executive Professor Sanchia Aranda has put her weight behind immunotherapies more broadly and their significance in treatment standards.
“It has been on the research agenda for a long time because we know that all people produce cancer cells every day. We all create faults in our cells that our immune system can mop up but those with the disease can’t work fast enough.”
‘Immunotherapy is a sniper’
Unlike chemotherapy, radiotherapy or targeted therapy – immuno-oncology goes to the source.
“In comparison, traditional treatments like chemotherapy are a bomb, wiping out all of a patient’s cells regardless of whether they’re good or bad,” she said.
“Immunotherapy is a sniper – targeting only particular proteins associated with the cancer.”
But it is pricing of the drugs that is holding back what some have called “miracle treatments”.
“In melanoma we have seen patients with metastatic conditions, with little chance of survival, completely get rid of the disease,” she said.
“Better results have only increased peoples’ willingness to pay and now it can cost in excess of $100,000 a year to treat.”
Prima have already partnered with big name pharma companies Novartis and Glaxosmithkine for two of their LAG-3 treatments, and are making modest royalties from their developments.
Their share price was described as frustrating by chairman Lucy Turnbull in their annual report this year, a sentiment echoed by the CEO.
“We have strongly retail driven stock, but relatively technical communication,” he said.
“In biotechs you have to have the endurance to weather share prices going up and down. Every clinical trial that goes wrong in the industry is a normal event – just a scientific experiment that doesn’t mean that your drug is not effective but in biotech you do not always have a second shot at goal.”