Medibio shares are recovering after a big fall yesterday when the CEO resigned

Pic: Godji10 / iStock / Getty Images Plus via Getty Images
Shares in mental health tech company Medibio plummeted 40 per cent on Wednesday afternoon after the company announced its managing director and CEO Jack Cosentino would depart the company immediately.
Melbourne-based Medibio (ASX:MEB) uses biometrics to screen, diagnose, monitor and manage depression and other mental health conditions.
The stock went into a trading halt on Tuesday investors were told on Wednesday that Mr Cosentino would cease his roles effective immediately.
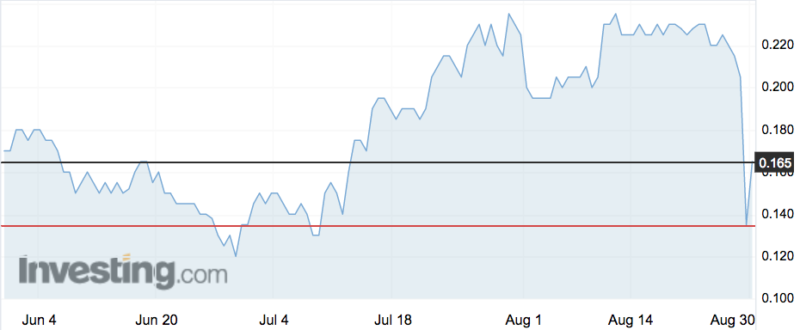
The shares fell 35 per cent to 13.5c — but recovered to 15c today on the back of full-year results, which revealed sales had risen 786 per cent year-on-year, though overall revenue took a hit thanks to a decreased R&D tax rebate.
Chairman Chris Indemaur declined to comment on the reasons behind Mr Cosentino’s departure, but said he left with the company’s best wishes.
“We wish him well. There is no hostility, Jack has done a lot of great work. Some things Jack did really well and he assisted the company through its recent phase of growth,” Indemaur told Stockhead this morning.
“There is no change to the substance of the company. We’ve lost our frontman and we are now searching for a new CEO and we start that process straight away.”
Brian Mower, the company’s CFO, has been appointed as interim CEO as it searches for Mr Cosentino’s replacement.
Mr Indemaur said the board supported Mr Cosentino’s departure.
“The company will remain focused and steadfast on its continued mission in identifying the link between physiological measures and mental health.
“We are fortunate to have the experience and dedication of Brian to move the company forward in the right direction while we embark on our search for a new CEO.”
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
Mr Cosentino, who had previously held senior positions at ImpediMed, Diversified Medical Corp and Medtronic, was appointed as CEO to much fanfare in February last year.
In May of this year, he oversaw the company winning approval to sell its stress-testing products in Europe.
The company’s loss also ballooned to $16.3 million, up from $9.8 million the year before.
In the financial year the company raised $13.9 million, completed an FDA clinical study and got approval from the TGA.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








