It’s renovation time at Bionomics after *that* failed trial

Drug developer Bionomics is making major changes after the failure of a key clinical trial in October, unveiling a $7.9 million capital injection, a board restructure, and a strategic review.
Bionomics (ASX:BNO) snuck out two major announcements on Friday after the market had closed then put its shares in a trading halt before it opened on Monday morning. The halt is expected to last until at least Wednesday.
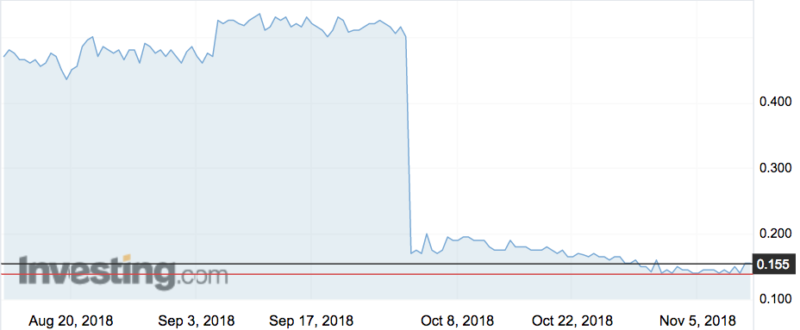
The upheaval follows the failure of the company’s BNC210 drug to treat patients with post traumatic stress disorder. The fizzled trial caused BNO shares to free fall from 50c to 17c last month.
The first of the changes has already happened: Dr Deborah Rathjen has retired as managing director, although she will remain as CEO until January 31 next year.
Dr Errol De Souza, currently a non-executive chairman, is now executive chairman.
An ‘executive’ director works within the business as well as sitting on the board.
Steven Lydeamore, the current CFO, is resigning and will be replaced by pervious CFO Stephen Birrell in the interim.
The failure of BNC210 will be the focus of a strategic review to be undertaken by Greenhill & Co.
The company also announced a recapitalisation, which is a restructuring of a company’s debt and equity mixture in order to stabilise its capital structure.
It was led by Bionomics’ major shareholder BVF Partners, which bought more than 48 million shares at 16 cents a pop to raise nearly $7.9m. BVF now owns just under 20 per cent.
Dr De Souza said the changes were necessary to ensure the company’s future.
“In order to preserve and enhance shareholder value, we are continuing to assess our strategic options for partnering and portfolio prioritisation and protection of our major assets whilst continuing to implement further cost-cutting measures to conserve cash,” he said.
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
“The change in leadership reflects the smaller organisation that Bionomics has become. Deborah has been pivotal in building Bionomics from its inception as a genetics company in 2000 to developing a strong therapeutics portfolio through both an acquisition and internal development strategy.
“She was instrumental in implementing our partnering strategy which has resulted in multiple collaborations over the years.”
Dr De Souza said they were “disappointed” that the phase 2 PTSD trial failed and now they’ll focus on a phase 2 trial of the BNC210 drug in hospitalised, elderly patients suffering from agitation.
“We have stopped all other work on BNC210 until that time. We anticipate that the strategic review will be completed by the end of the first calendar quarter of 2019, following completion of the BNC210 trial and possible announcements related to advancement of the cognition program licensed to MSD.”
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.