Bionomics just announced a failed trial and its shares are in free fall
Health & Biotech
Health & Biotech
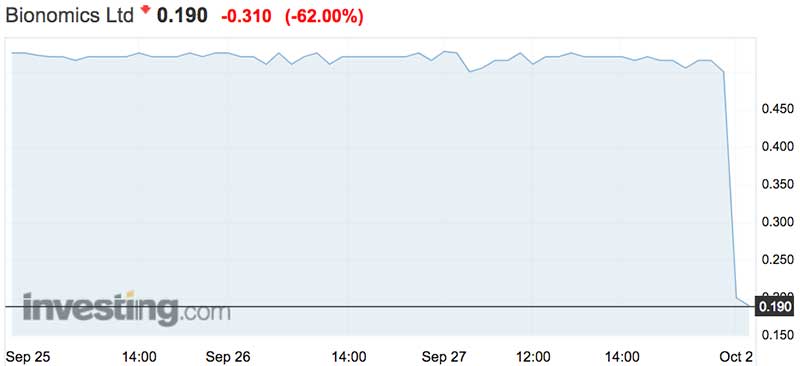
Bionomics is in free fall after announcing a trial of its Post Traumatic Stress Disorder drug failed to reduce symptoms after 12 weeks.
The stock has tanked 68 per cent in the opening minutes of trade on Tuesday morning to 16c.
The phase two trial assessed PTSD symptoms in 193 patients across 25 sites in the US and Australia after they’d been treated with BNC210, a new drug that inhibited a neural receptor implicated in long term memory.
“We are extremely disappointed that the primary endpoint in this trial was not met,” said Bionomics chief Dr Deborah Rathjen.
The drug did not show an effect when patients were assessed under the CAPS-5 (clinically administered PTSD scale) system, a 30-item questionnaire used by clinicians to assess symptoms.
Bionomics (ASX:BNO) consultant chief medical officer Professor Paul Rolan said they saw improvements on some components of the scale that are attributable to the mood and anxiety.
The trial proved the drug was safe to use in humans, even if it didn’t work, however.
“There was great anticipation that it would show clear beneficial effects on improving PTSD symptoms,” said consultant Dr. Murray Stein, distinguished professor of Psychiatry, Family Medicine and Public Health at the University of California San Diego.
He said the phase two trial results didn’t demonstrate those benefits.
He said that underscored “the complexity of PTSD and the heterogeneity of PTSD symptoms across patients”.
The company is moving forward with a phase 2 trial of the drug in elderly patients in hospitals who are suffering from agitation, expected to end in the first quarter of 2019.
Dr Rathjen says all other work on BNC210 is now suspended and they’re assessing their options for their drug candidate portfolio.
Bionomics is not a one-drug pony however, so all is not lost.
“Bionomics has ongoing drug discovery programs which are anticipated to deliver up to two new therapeutic candidates this financial year,” Dr Rathjen said.
“We also have a strategic partnership with Merck & Co, assessing a cognition therapy candidate in an ongoing Phase 1 program, which entered clinical development and triggered a US$10 million milestone payment last year.”