Encouraging signs for MGC Pharma after cannabinoid treatment for aggressive brain cancer breakthrough
Health & Biotech
Health & Biotech
MGC Pharma is working to provide treatment hope for one of the most common and aggressive forms of brain cancers with an early study showing positive signs they’re on the right track.
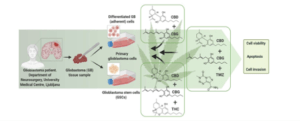
Aussie-listed biopharma MGC Pharmaceuticals (ASX:MXC) has announced success in an in-vitro preclinical research study into the use of cannabinoids to treat glioblastoma multiforme cells, a fast growing and aggressive form of brain cancer.
Based in Europe and specialising in production and development of phytomedicines, the glioblastoma research study was undertaken in collaboration with the National Institute of Biology in Slovenia.
Conducted between 2019 and 2022, the study initially used a formulation which included THC (delta-9-tetrahydrocannabinol), which was later replaced with cannabigerol (CGB), which has no known psychotropic effects.
The study was undertaken on 30 biopsy samples from 18 patients and more than 5,800 cell tests over the course of the study to determine the most effective concentrations and ratios of cannabidiol (CBD) and CGB in the treatment formulation.
Results of the study have demonstrated the efficacy of cannabinoids in treating glioblastoma, one of the most common types of malignant brain tumour in adults, MCG Pharma says.
The study also determined the most effective ratio of CBD:CBG in inhibiting the tumours’ viability, setting off the cascade of biological processes leading to the apoptosis (cell death) of the glioblastoma tumour and stem cells.
With glioblastoma stem cells are the main cause of the disease’s progression, and highly resistant to standard therapies.
MGC Pharma is also undertaking studies in collaboration with UK company, Graft Polymer UK PLC, on the use of a base formulation nano delivery system.
Based on Graft Polymer’s Graft-Bio IP to improve bioavailability of the active compounds using a non-invasive drug administration process, the studies are examining toxicity of the base emulation to confirm its safety profile for potential use in future clinical research undertaken by MGC Pharma.
The study also examined the effect of adding a widely used chemotherapy agent to the treatment formulation to determine the effect on its efficacy.
Results demonstrated that the addition of chemotherapy bore no contributing effects to cell apoptosis.
Significantly, the results suggest that MGC Pharma’s cannabinoid treatment can potentially be used as a treatment in cancer patients without the inclusion of toxic agents.
Results will be the subject of further research in studies to determine the efficacy of MGC’s glioblastoma treatment formulation in a clinical trial setting.
The treatment formulation’s advancement to the next stage of the clinical trial process is anticipated to provide a crucial addition to MGC Pharma’s intellectual property.
MGC Pharma co-founder and managing director Roby Zomer says the results of this trial are enormously exciting both for the company, and for treatment of fatal cancerous tumours.
“MGC Pharma’s research has demonstrated the effect of naturally derived cannabinoid products on stage IV brain tumours without the use of toxic chemotherapy components.”
Zomer says it will also be a transformative step forward in the treatment of aggressive brain cancers.
“We are proud of the work achieved thus far and are looking forward to advancing our proprietary formulation to the next stage of clinical trials.”
Meanwhile, MGC’s Pharma’s latest clinical trials have shown to reduce aggressive behaviour in dementia patients, while also demonstrating the product’s ‘full safety and preliminary efficacy’.
This article was developed in collaboration with MGC Pharmaceuticals, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.