MGC Pharma results provides real hope for Alzheimer’s sufferers
Health & Biotech
Health & Biotech
This could be a big win for dementia sufferers, as Aussie-listed biopharma MGC Pharmaceuticals (ASX:MXC) says its latest clinical trials have shown to reduce aggressive behaviour in patients, while also demonstrating the product’s ‘full safety and preliminary efficacy.’
While it’s still early days, MGC Pharma’s recently completed Phase II Clinical Trial for its proprietary Dementia treatment, CogniCann could provide some desperately sought relief not only for dementia sufferers but their families.
According to Dementia Australia, this year, some 1.6 million Australians are involved in the care of someone living with dementia which is – unbelievably – the second leading cause of death here.
It’s the leading cause of death for Aussie women. And the consequent burden of disease on everyone impacted is enormous, shocking even.
So there’ll be a fair bit of interest with MXC saying these phase II Clinical Trial results demonstrate the product’s ability to inhibit the deterioration in the behaviour of dementia patients – and a lot more.
MGC Pharma is a specialist in the production and development of phytomedicines, developing the Investigational Medicinal Product, (IMC) CogniCann as an oral spray containing delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) properties designed to improve behaviour and cognition in dementia patients.
In detailed results data provided to the exchange, the biopharma says the study in conjunction with experts at the University of Notre Dame in Western Australia assessed the safety and efficacy of CogniCann, including a focused assessment of the products behavioural benefits on Dementia patients.
The double-blind study’s protocol provides a fascinating breakdown of the evaluation tools employed including a Neuropsychiatric Inventory – Nursing homes (NPI-NH) Questionnaire and a Cohen-Mansfield Agitation inventory Questionnaire.
Certainly the positive impact on aggressive behaviour – one of the most serious of the many disturbances experienced by dementia patients is itself a cause for hope, if not celebration.
Aside from the obvious cost in distress, the unpredictable aggressive behaviour in sufferers is the most common cause for psychiatric referral, admission to hospital and drug treatment.
During the 44-day study period the treatment group’s Cohen-Mansfield Agitation Inventory Aggressive subscale improved by 13%, compared with the Placebo group which improved by 4%.
This important finding indicates not only improvement in the health status of the patients, but also the improved quality of life of the families and caregivers that are taking care of dementia patients.
The graph below demonstrates the change in the aggressive behaviour between the Treatment and Placebo groups.
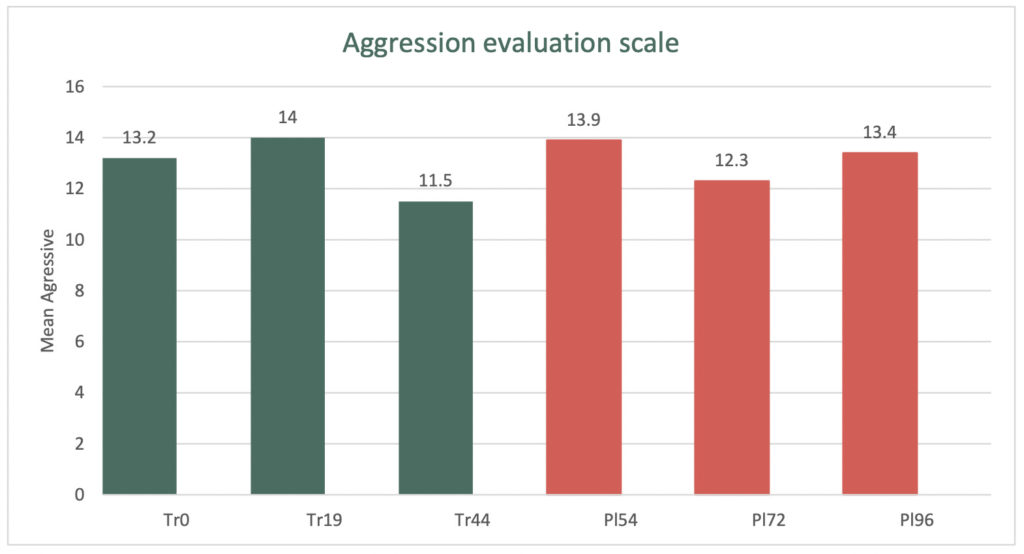
The double-blind cross-over Clinical Trial involved eligible patients commencing a six-week treatment course, before switching (crossing over) to a six-week course of a placebo, with a two-week ‘washout’ period between the two arms.
Results from the study will be used in the design of the next phase of clinical trials including defining the appropriate End Points and patient sample size. The data will also be considered as a vector and will be studied in future clinical trials using the larger sample size.
Which unfortunately will not be difficult to source.
According to the World Health Organisation, someone in the world develops dementia every three seconds.
The WHO says back in 2020 there was some 55 million people worldwide living with the disease, although that figure will just about double every 20 years, topping 78 million by 2030 and 139 million in 2050.
Much of the increase will be in developing countries. Back in April, MGC Pharma signed a supply and distribution agreement with Sciensus Rare for the distribution of CogniCann in key European markets.
The fastest growth in the elderly population is taking place in our own region. Across China, India, and in the south Asian and western Pacific regions.
In 2022 according to Dementia Australia there’s circa 500,000 Australians living with dementia, but without a medical breakthrough, that’s expected to increase to almost 1.1 million by 2058
Of growing concern as well – there’s an estimated 28,800 people with younger onset dementia, of course that’s expected to rise. And while 70% of people with dementia live in the community, more than two-thirds (68.1%) of aged care residents have moderate to severe cognitive impairment.
This article was developed in collaboration with MGC Pharma, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.