Cancer-fighter Race Oncology jumps 120pc after putting its drug up for sale

Cancer biotech Race Oncology more than doubled today after starting the hunt for a “sale, licence, partnership, collaborative venture or other arrangement” for its drug Bisantrene.
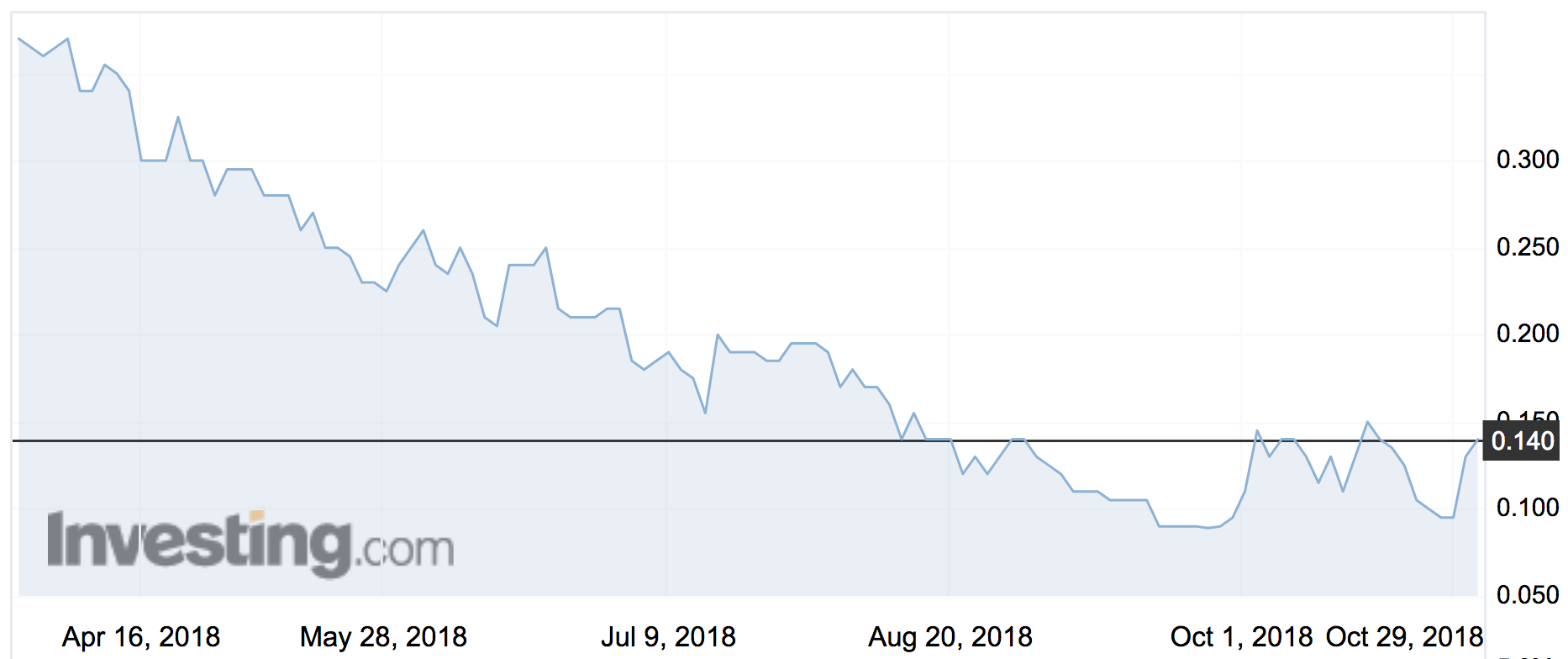
Race’s (ASX:RAC) shares shot up 123 per cent to an intraday high of 23.5c.
That’s good news for shareholders who’ve watched the share price steadily decline from around 50c at the start of the year.
Race has signed up New York-based Biosynergy Partners, owned by one of Race’s directors, to look for deals for the drug.
While it would consider any kind of offer, Race was particularly interested in licensing deals that offloaded development costs to another party — and where “upfront licence fees” were paid.
Race chief Peter Molloy says this is the first step they’ve take towards finding a deal.
“We recently announced a consulting agreement with Tom Lee that is focused on potential funding and clinical partnerships in Houston Texas, with a focus on clinical partnerships with MD Anderson Cancer Center. The Biosynergy agreement is focused on licensing of Bisantrene to commercial partners,” he told Stockhead.
Bisantrene is a chemotherapy drug that was tested in more than 40 clinical studies before it was “lost” in a series of pharmaceutical mergers in the 1990s.
There haven’t been many licensing deals this year among small cap biotechs, and particularly in the cancer field.
Of the few, there’s been Neuren Pharma (ASX:NEU) signed a deal with NASDAQ-listed ACADIA Pharma to develop and commercialise its Rett syndrome treatment, a debilitating neurological disorder that occurs primarily in baby girls.
And Benitec (ASX:BLT) signed a deal with Axovant Sciences for its oculopharyngeal muscular dystrophy drug.
Drug “rescue”
Race says it’s “rediscovering and rescuing” Bisantrene to treat and the initial clinical opportunity is for treatment of relapsed AML — a kind of leukaemia.
It has orphan drug status in the US, a designation that incentivises the creation of drugs for rare, otherwise commercially non-viable diseases.
“We are particularly interested in exploring licensing deals, where the partner carries the cost of the proposed AML registration trial,” said RAC chief Peter Molloy.
“We would also like to see upfront license fees paid that would represent non-dilutive funding for Race.”
- Subscribe to our daily newsletter
- Bookmark this link forsmall cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








