Zelira’s primed to launch the world’s first clinically validated cannabis product for insomnia

Zelira Therapeutics (ASX:ZLD) will launch the world’s first clinically validated medicinal cannabis product for insomnia later this year after a positive clinical trial result.
The company’s ZLT-101 formulation was tested in 23 patients with chronic insomnia. The drug met the primary endpoints of showing a statistically significant improvement in the Insomnia Severity Index (ISI) scores as well as safety and efficacy.
The ISI is not imsomia itself. It is a survey that assesses the severity of insomnia side-effects in patients formally diagnosed with insomnia. But it is the current gold standard to measure efficacy of medicines to treat insomnia.
According to Professor Peter Eastwood, the principal investigator of the study, it was the most rigorous clinical trial ever undertaken to test cannabis as a treatment for insomnia.
“These results suggest ZLT-101 has potential as a novel treatment for insomnia,” he said.
Dr Richard Hopkins, managing director ex-US markets, dubbed the results “the best outcome you could imagine”.
“It’s a really important milestone, it cements our global leadership position in the field and delivers on our commitment to deliver clinically verified cannabis medicines,” he said.
“We’re well positioned to launch the world’s first treatments into global markets in 2020.
“It validated the confidence we had and our commitment to getting them to the market.”
Dr Hopkins added that this data meant doctors could now be confident in prescribing medical cannabis.
“We [have] given physicians a reason to prescribe this compound — clinical evidence,” he said. “There are very few [cannabis] products that can do that today if any.”
The news pushed shares up 7 per cent this morning.
What now?
The company is now awaiting the final report on whether the trial met the secondary endpoints. This is due for release by the end of next month.
Dr Hopkins told Stockhead on this morning’s conference call the final report will include details such as the quality and length of sleep.
“That’s going to be powerful, they will allow us to understand how [ZLT-101] sits alongside existing therapies and address what they don’t do,” he said. “They’ll provide a lot of colour.”
When Zelira does launch these products in America, it said it would be entering a large market. There are 70 million insomnia patients in the US alone and they spend $US2bn ($3bn) on insomnia medications.
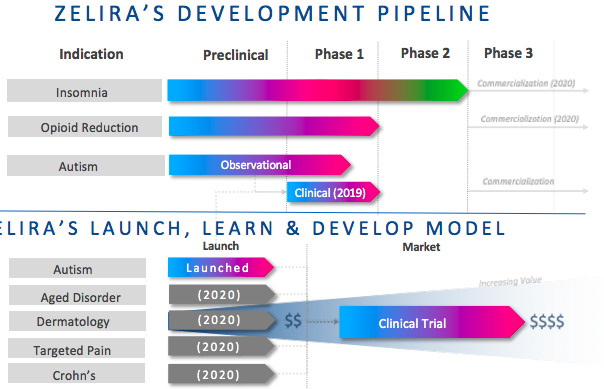
The company has two other trials underway — in opioid dependence reduction and autism. Others are planned to be launched in the coming months.
Related Topics
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








