Weed Week: MDMA for PTSD hits roadblock with FDA; and recent ASX cannabis stock winners
* FDA panel rejects MDMA for PTSD treatment, citing safety and data concerns. Picture Getty
- FDA panel rejects MDMA for PTSD treatment, citing safety and data concerns
- Advocates argue MDMA’s benefits outweigh risks, call for further research.
- South Africa legalises personal cannabis use, setting precedent for African nations
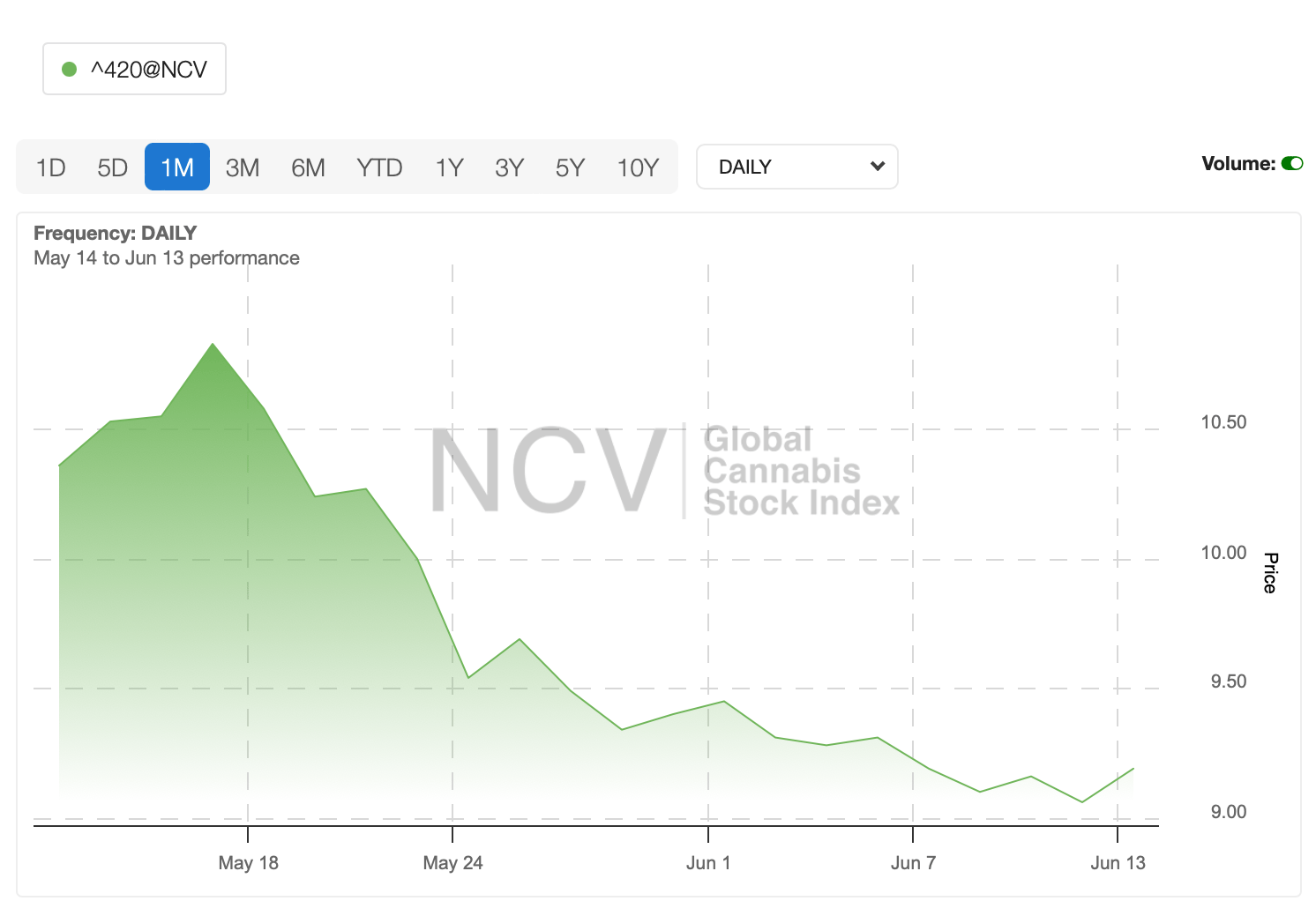
Global weed stocks have been on bumpy ride over the past month, with the cannabis index down around -11%.
Last week’s decision by a US FDA advisory panel to reject MDMA (commonly known as ecstasy) as a treatment for PTSD has stirred debate and disappointment among researchers and advocates.
The panel, consisting of experts in psychiatry and drug safety, expressed reservations on the clinical study conducted by unlisted US biotech, Lykos Therapeutics, which is seeking FDA approval for MDMA as a treatment.
Concerns voiced by panel members centred around the safety profile of MDMA, particularly regarding potential long-term neurological and psychiatric effects.
Some also raised questions about the consistency and reliability of Lykos’ trial data, noting variability in study designs and outcomes.
Dr Melissa Decker Barone, a psychologist with the Department of Veterans Affairs, said there were too many problems with Lykos’ data.
“Each one alone might be OK, but when you pile them on top of each other … there’s just a lot of questions I would have about how effective the treatment is,” said Dr Decker Barone said.
Advocates for MDMA therapy however argue that the benefits observed in the trials outweigh the potential risks, especially for individuals who have not responded to conventional treatments.
They emphasise the need for more research to address the safety concerns and refine treatment protocols, instead of rejecting MMDA outright.
Doug Drysdale, the CEO of Cybin, a clinical-stage, psychedelic-based therapies biotech, says that while this news might seem like a setback, many of the issues raised by the advisory committee were known and not a surprise to the FDA or to most followers of the sector.
“It seems clear that there is strong regulatory support for psychedelic drug development from the agency, and the many issues raised were specific to the data set that was submitted,” Drysdale said.
“In many ways, the protocol under scrutiny by the advisory panel is an outlier in the sector. “
MDMA – also known as ecstasy – is paving the way for a wave of psychedelics, such as LSD and psilocybin, to undergo FDA evaluation in the near future.
There’s a hopeful buzz around these substances, with advocates believing they could revolutionise how we approach mental health disorders.
The FDA typically follows the panel’s advice, but it’s not required to. The final decision is expected in August.
On the ASX, one company that focuses on MDMA is Emyria (ASX:EMD).
South Africa finally embraces cannabis
Meanwhile, South Africa has made a groundbreaking move this week by legalising the use of cannabis for personal purposes.
The decision marks a significant shift in the country’s stance on marijuana (also called ‘dagga’ in the country), and could potentially serve as a model for other African countries considering similar reforms.
The new law basically allows adults to possess and use cannabis in private spaces. However, it maintains restrictions on public use and selling to minors.
This decision comes after years of debate and legal battles, with proponents arguing for the benefits of legalisation, including economic opportunities and reduced strain on the criminal justice system.
South Africa’s decision reflects shifting attitudes toward cannabis worldwide and could pave the way for similar reforms across the African continent.
Some surrounding countries in the region have already taken steps toward decriminalisation or legalisation, while others remain staunchly opposed.
To ASX Weed Stocks ….
Here’s how the ASX weed stocks have performed, sorted by winners over the past month.
| Code | Company | Price | % Week | % Month | % 6-Month | % Year | Market Cap |
|---|---|---|---|---|---|---|---|
| IDT | IDT Australia Ltd | 0.125 | 22% | 35% | 35% | 97% | $43,934,934 |
| ALA | Arovella Therapeutic | 0.140 | 17% | 17% | 47% | 204% | $147,021,873 |
| DTZ | Dotz Nano Ltd | 0.145 | -3% | 12% | 0% | -42% | $75,945,506 |
| LV1 | Live Verdure Ltd | 0.445 | 2% | 6% | 41% | 230% | $55,560,829 |
| BOT | Botanix Pharma Ltd | 0.285 | 2% | 4% | 104% | 191% | $448,914,129 |
| EMD | Emyria Limited | 0.051 | -12% | 2% | -9% | -60% | $20,858,459 |
| BOD | BOD Science Ltd | 0.024 | 0% | 0% | 0% | -49% | $4,256,124 |
| HGV | Hygrovest Limited | 0.046 | 0% | 0% | -10% | 0% | $9,674,288 |
| ROO | Roots Sustainable | 0.007 | 0% | 0% | 0% | 40% | $1,124,217 |
| EXL | Elixinol Wellness | 0.004 | 0% | 0% | -61% | -66% | $5,284,729 |
| EPN | Epsilon Healthcare | 0.024 | 0% | 0% | -11% | 20% | $7,208,496 |
| AC8 | Auscann Grp Hlgs Ltd | 0.040 | 0% | 0% | 0% | 0% | $17,621,884 |
| MDC | Medlab Clinical Ltd | 6.600 | 0% | 0% | 0% | 0% | $15,071,113 |
| EVE | EVE Health Group Ltd | 0.001 | 0% | 0% | 0% | 0% | $5,274,483 |
| WNX | Wellnex Life Ltd | 0.024 | -14% | 0% | -40% | -40% | $30,949,304 |
| AVE | Avecho Biotech Ltd | 0.003 | -25% | 0% | 0% | -50% | $9,507,891 |
| VIT | Vitura Health Ltd | 0.096 | 1% | -2% | -65% | -75% | $55,283,884 |
| ECS | ECS Botanics Holding | 0.017 | 6% | -6% | -23% | -11% | $21,905,343 |
| EOF | Ecofibre Limited | 0.051 | -39% | -6% | -51% | -75% | $19,322,569 |
| NTI | Neurotech Intl | 0.063 | 11% | -15% | 11% | 58% | $64,095,481 |
| LGP | Little Green Pharma | 0.110 | -8% | -15% | -19% | -35% | $33,193,667 |
| AGH | Althea Group | 0.022 | -19% | -15% | -39% | -51% | $8,917,314 |
| BP8 | Bph Global Ltd | 0.004 | 33% | -20% | -60% | -77% | $1,563,299 |
| CAN | Cann Group Ltd | 0.048 | 0% | -23% | -54% | -65% | $21,387,655 |
| IRX | Inhalerx Limited | 0.030 | 0% | -25% | 0% | -30% | $5,693,009 |
| WFL | Wellfully Limited | 0.003 | -25% | -25% | -25% | -67% | $1,478,832 |
| RGT | Argent Biopharma Ltd | 0.300 | -6% | -26% | -41% | -95% | $13,584,061 |
| ZLD | Zelira Therapeutics | 0.440 | -4% | -34% | -53% | -74% | $4,992,748 |
| WOA | Wide Open Agricultur | 0.019 | -5% | -37% | -89% | -94% | $4,246,945 |
| ME1 | Melodiol Glb Health | 0.021 | -19% | -77% | -98% | -100% | $576,442 |
Botanix has this week announced a major milestone in its journey towards FDA approval for Sofdra, a treatment for excessive underarm sweating.
The company said it has submitted final label materials to the US FDA, marking the last step before potential approval.
These label discussions with the FDA have focused on designing the product carton and refining the information that will be provided to patients and healthcare providers.
The materials submitted include important documents like the Prescribing Information and Patient Information.
The FDA is expected to make a decision on Sofdra by June 21, 2024. If approved, Sofdra will be the first new treatment approved specifically for primary axillary hyperhidrosis, addressing a significant medical need.
IDT confirmed it has received a non-binding proposal from Myndbio Pty Ltd regarding a potential acquisition of all IDT shares.
Discussions are ongoing, with Mynd granted access to conduct due diligence under standard confidentiality terms.
Mynd has proposed a non-binding indicative cash offer of up to $0.15 per IDT share.
If the proposal moves forward, it would likely be through a scheme of arrangement, subject to conditions including successful due diligence and board approvals.
However, these discussions are preliminary, and there’s no certainty they will lead to a binding agreement or an offer within a recommended price range by IDT’s board.
Arovella Therapeutic (ASX:ALA)
Arovella has been riding high after successfully developing a patented manufacturing process for its lead product, ALA-101, designed for large-scale production under Good Manufacturing Practice (GMP) standards.
This modular and semi-automated process, developed at Cell Therapies, is capable of producing high quantities of Chimeric Antigen Receptor (CAR)-positive iNKT cells with exceptional purity.
This is a significant milestone as it establishes a reliable GMP manufacturing process crucial for gaining regulatory approval to conduct initial human trials.
With this achievement, Arovella can now move forward with engineering and producing GMP batches needed for phase 1 clinical trials.
Related Topics
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.