Weed Week: A VAST majority of Americans now want marijuana to be legal

Here’s a quick recap of the cannabis sector in the US and Australia. Picture Getty
- Where are we in terms of US legalisation of cannabis?
- What about the current legal landscape for cannabis in Australia?
- We look at the best performing ASX weed stocks this past month
Global weed stocks have been on bumpy ride over the past month, driven mainly by global geopolitical events.
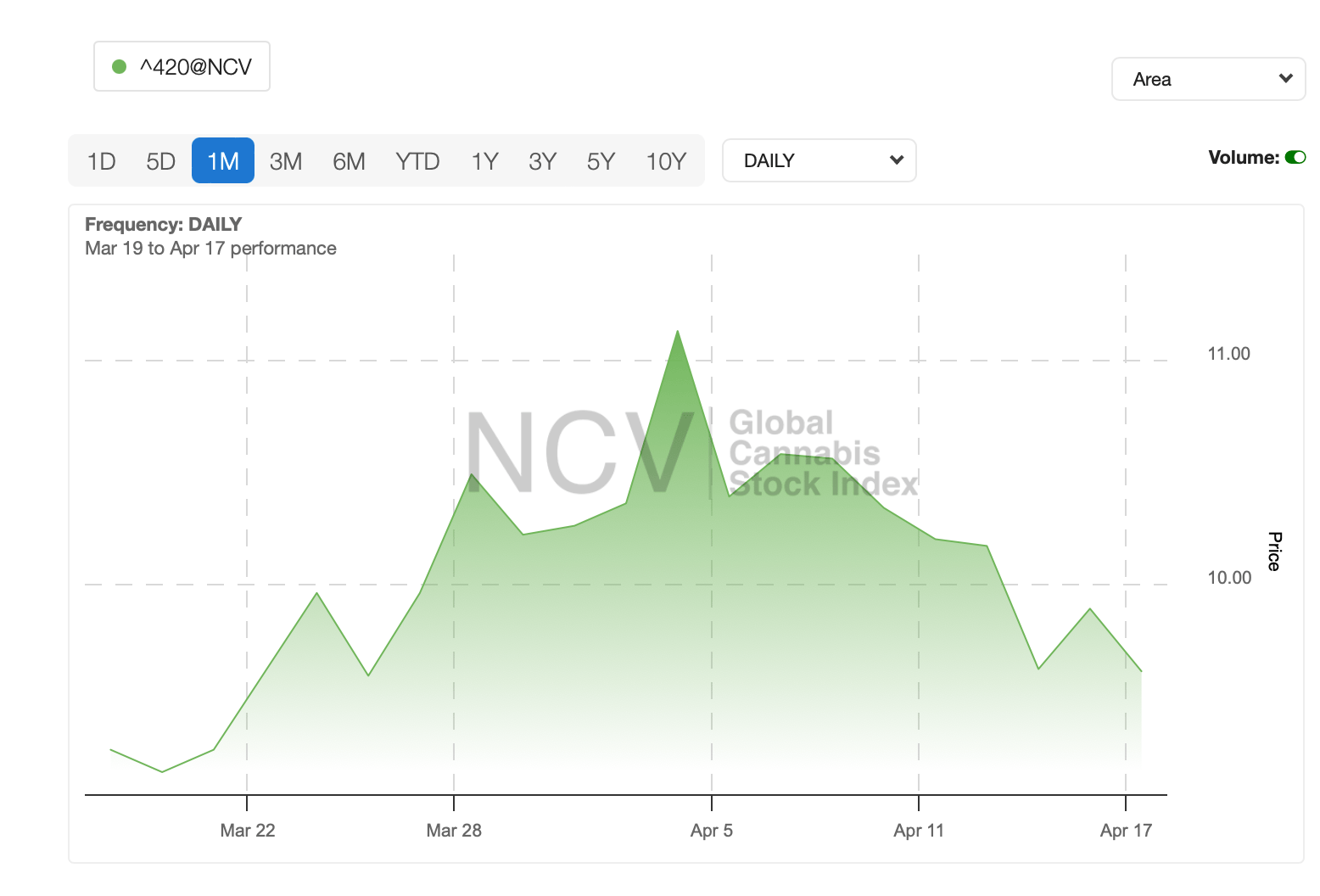
As we enter Q2, it’s probably a good time now to give a quick recap of what’s happening in the US in terms of cannabis legalisation.
As a reminder to readers, on August 31 2023 the US Department of Health and Human Services (HHS) requested the Drug Enforcement Agency (DEA) to consider easing restrictions on marijuana to a Schedule III drug.
Schedule III drugs are defined as “drugs with a moderate to low potential for physical and psychological dependence.”
If the DEA accepts HHS’s recommendation, it could ease marijuana access and spur an industry hemmed in by US federal regulations, even as restrictions have begun to ease at state level.
As of today however, there has been no definitive development and we’re still watching to see how that will shake out.
Read more here: Growing excitement for cannabis stocks after marijuana rescheduling hopes in the US
To summarise, the use and possession of marijuana in the US is currently illegal at the federal level.
However, survey after survey have indicated that the vast majority of Americans (around 90%) want marijuana to be legal.
The latest Pew Research data shows that about three-quarters of US states have legalised the drug for either medical or recreational purposes.
These include 24 states and the District of Columbia, which have legalised small amounts of marijuana for both medical and recreational use.
Another 14 states have legalised the drug for medical use only.
In total, about 8 in 10 Americans (79%) now live in a county with at least one cannabis dispensary.
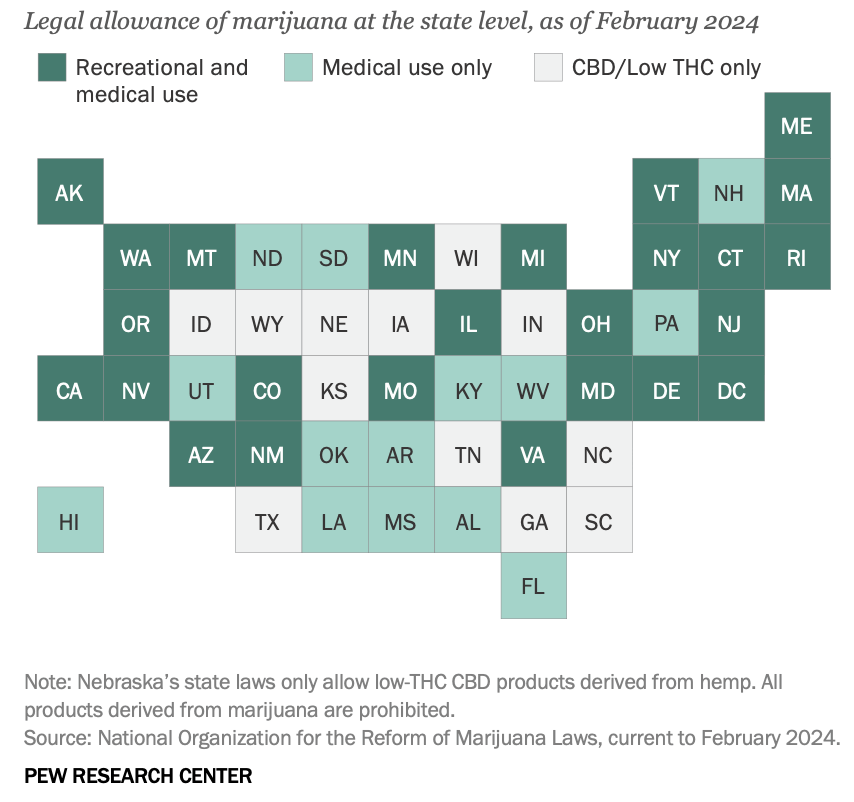
“Of the remaining 12 states, all allow limited access to products such as CBD oil that contain little to no THC – the main psychoactive substance in cannabis,” said Pew Research.
So where do we go from here?
Most experts predict the DEA would follow through with HHS’ request, the reason being that the DEA has to rely on the HHS for scientific and medical information, which strongly influences scheduling.
“I would be really shocked if it took the DEA longer than the second quarter of 2024 to come up with its final rule,” said Howard Sklamberg, a former FDA official.
The upcoming US election will also help determine legalisation at the federal level, and the future of cannabis reform in 2025 and beyond.
“If Trump or another Republican takes the White House — or if Republicans hold the House or win a Senate majority—there’s virtually no chance for federal legalisation,” said weed expert, Alexander Lekhtman.
What about cannabis reforms in Australia?
Cannabis is still largely illegal in Australia, but the rules are different from state to state.
Here’s a state by state status for cannabis:
NSW: Supply and possession of cannabis is illegal, but first-time offenders with less than 15 grams can get off scott-free with only a warning. However, any approved doctor in NSW can prescribe medicinal cannabis if it was determined to be an appropriate treatment for the patient.
VIC: First-timers caught with 50 grams in possession are given a caution and will be requested to attend counselling. As with NSW, medicinal cannabis can be prescribed by an approved doctor for patients that have no other medical alternatives.
QLD: Possession, production and trafficking of cannabis can get you up to 20 years imprisonment. Medicinal cannabis, on the other hand, can be prescribed by approved doctors.
SA: Keeping, growing, using or giving marijuana is illegal in South Australia, with penalties of up to $1m and 15 years jail. Medical cannabis however can be bought, but only via prescription from authorised medical practitioner and dispensed by a pharmacist.
WA: People who possess 10 grams or less can avoid criminal conviction, but more than 10g can get you a fine of up to $2,000 or 2 years in prison. Medicinal cannabis is available via prescription from any authorised doctor in WA.
TAS: In short, possession of cannabis is illegal in Tassie. For medical cannabis, GPs can prescribe cannabis treatment to patients if they deem it appropriate.
NT: Cannabis is largely decriminalised in NT, however large possessions can still get you in hot water. Medicinal cannabis however can be prescribed to patients from an authorised practitioner.
What’s TGA’s stance on medicinal cannabis?
The TGA’s (Therapeutic Goods Administration) stance on medicinal cannabis is the following:
“While the TGA regulates the access, most medicinal cannabis products are considered to be unapproved medicines. Unapproved medicines have not been assessed by the TGA for safety, quality or effectiveness.
“The Australian Register of Therapeutic Goods (ARTG) Act provides a number of mechanisms, including the Special Access Scheme (SAS) and Authorised Prescriber (AP) pathway, to enable access to ‘unapproved’ therapeutic goods, such as medicinal cannabis.
“It is expected that registered health practitioners (prescribers) will have considered clinically appropriate treatment options that are included in the ARTG before applying to access an unapproved medicinal cannabis product under the SAS or AP pathway,” noted the TGA.
To ASX Weed Stocks ….
Here’s how the ASX weed stocks have performed, sorted by winners over the past month
| Code | Company | Price | % Week | % Month | % 6-Month | % Year | Market Cap |
|---|---|---|---|---|---|---|---|
| LV1 | Live Verdure Ltd | 0.835 | 38% | 56% | 109% | 406% | $97,735,177 |
| IDT | IDT Australia Ltd | 0.105 | 27% | 30% | 64% | 54% | $35,147,947 |
| BOT | Botanix Pharma Ltd | 0.220 | -2% | 19% | 63% | 129% | $322,903,145 |
| VIT | Vitura Health Ltd | 0.165 | 0% | 14% | -56% | -51% | $95,019,175 |
| ME1 | Melodiol Glb Health | 0.005 | -25% | 13% | -96% | -98% | $2,152,772 |
| LGP | Little Green Pharma | 0.140 | 4% | 12% | -15% | -22% | $40,526,762 |
| NTI | Neurotech Intl | 0.108 | 8% | 10% | 92% | 99% | $96,325,802 |
| AGH | Althea Group | 0.031 | 0% | 7% | -18% | -47% | $12,970,638 |
| AC8 | Auscann Grp Hlgs Ltd | 0.040 | 0% | 0% | 0% | 0% | $17,621,884 |
| AVE | Avecho Biotech Ltd | 0.004 | -11% | 0% | 0% | -20% | $12,677,188 |
| BOD | BOD Science Ltd | 0.024 | 0% | 0% | -57% | -61% | $4,256,124 |
| CAN | Cann Group Ltd | 0.062 | 0% | 0% | -48% | -61% | $27,123,891 |
| EPN | Epsilon Healthcare | 0.024 | 0% | 0% | -14% | 26% | $7,208,496 |
| EVE | EVE Health Group Ltd | 0.001 | 0% | 0% | 0% | 0% | $5,274,483 |
| EXL | Elixinol Wellness | 0.006 | 0% | 0% | 9% | -62% | $7,806,444 |
| HGV | Hygrovest Limited | 0.046 | 0% | 0% | -8% | -13% | $9,884,598 |
| MDC | Medlab Clinical Ltd | 6.600 | 0% | 0% | 0% | 0% | $15,071,113 |
| ROO | Roots Sustainable | 0.007 | 0% | 0% | 17% | -13% | $1,124,217 |
| RGT | Argent Biopharma Ltd | 0.430 | 0% | -1% | -79% | -96% | $19,696,888 |
| ZLD | Zelira Therapeutics | 0.710 | 1% | -3% | -25% | -31% | $8,169,952 |
| ECS | ECS Botanics Holding | 0.022 | -4% | -4% | -8% | 10% | $27,703,816 |
| WOA | Wide Open Agricultur | 0.100 | -13% | -9% | -69% | -39% | $17,902,342 |
| EMD | Emyria Limited | 0.057 | -8% | -11% | -16% | -65% | $21,631,134 |
| EOF | Ecofibre Limited | 0.070 | -10% | -18% | -56% | -59% | $26,521,173 |
| IRX | Inhalerx Limited | 0.040 | 0% | -20% | -7% | -11% | $7,590,678 |
| WNX | Wellnex Life Ltd | 0.020 | -23% | -20% | -50% | -61% | $25,005,226 |
| DTZ | Dotz Nano Ltd | 0.135 | -10% | -22% | -21% | -34% | $70,465,949 |
| WFL | Wellfully Limited | 0.003 | -25% | -25% | -25% | -73% | $1,478,832 |
| ALA | Arovella Therapeutic | 0.110 | 0% | -27% | 47% | 39% | $115,517,186 |
| BP8 | Bph Global Ltd | 0.001 | 0% | -33% | 0% | -80% | $1,954,116 |
IDT Australia (ASX:IDT) has entered into a Master Service Agreement with Sanofi, a global French healthcare company, to support the preclinical formulation development and cGMP manufacture of Sanofi’s messenger RNA (mRNA) for its clinical program.
The agreement allows Sanofi to choose services from IDT Australia and allows for follow-on work packages.
The value of the services to be provided under the initial order, which is nearing finalisation, is estimated to be between $3 to 3.5 million (excluding costs relating to storage, shipping and any equipment purchase)
Botanix Pharma has been included in the ASX All Ordinaries Index this week.
Botanix joins the ranks of the index, which comprises the 500 largest companies listed on the ASX, based on its strong performance over the last 12 months.
The performance of Botanix shares, which rose by +129% in the last 12 months, has been driven by interest in the ompany’s treatment for excessive underarm sweating that is expected to receive approval from the US FDA in late June.
In an Australian first, Vitura announced that its joint venture, Cortexa, has commenced batch manufacturing of GMP LaNeo MDMA 40mg capsules to support both clinical trials and clinical use under the TGA’s Authorised Prescriber pathway.
In doing so, Cortexa achieves another significant milestone, domestic Australian manufacturing, with further strengthens its position as a leader in the Australian psychedelic landscape by executing on its plan to develop a reliable local supply.
The company believes this advantage will free local clinicians and researchers from the costly and time-consuming burden of importation and provides seamless access to medication.
Melodiol Global Health (ASX:ME1)
Melodiol’s has generated $4.1 million in unaudited revenues for Q1 FY24, a 79% increase on the pcp, providing what it says is a ‘strong foundation for the remainder of FY24’.
Melodiol’s wholly-owned recreational cannabis subsidiary Mernova Medicinal was the main driver, recording $1.7 million in revenue for Q1 FY24 to build on the strong momentum generating by the division in FY23.
Mernova’s high-quality recreational cannabis products, sold under the Ritual brand, continues to experience robust demand across major Canadian provincial markets.
Mernova already has more than $550k worth of purchase orders secured for delivery in the Q2 FY24, providing considerable potential upside to round out H1 CY24.
AGH, through its wholly-owned subsidiary Althea MMJ UK, has entered into an exclusive distribution agreement with Just Brands International Ltd to become the sole supplier of Vessel branded vape pens and accessories for the UK medical market
Vessel’s premium products fill a market gap for reusable vape pens and accessories, serving the increasing number of UK medical cannabis patients.
MyAccess Clinics, a leading medical cannabis clinic in the UK and wholly-owned subsidiary of AGH, will commence selling Vessel branded products from this month.
Vessel product sales will be purely accretive to the company, requiring no inventory carrying costs, says AGH.
Neurotech International (ASX:NTI)
Neurotech has treated the last patient in its Phase II/III NTIASD2 clinical trial for children with Autism Spectrum Disorder.
The trial, aiming to confirm the therapeutic effects of NTI164 observed in previous trials, seeks to address the urgent need for new treatments in Australia, where autism prevalence is rising.
The study comprises an eight-week treatment period, followed by an eight-week open-label maintenance period, followed by a two-week wash-out period.
Participants who choose to continue receiving NTI164 beyond the duration of the study may do so for an additional 38 weeks. They will undergo the two-week down-titration phase at the end of their extension phase.
The primary endpoint of the trial is the Clinical Global Impression-Severity (CGI-S), which reflects a clinician’s impression of severity of illness on a 7-point scale ranging from 1 = “not at all” to 7 = “among the most extremely ill”.
At Stockhead we tell it like it is. While Melodiol Global Health and Neurotech are Stockhead advertisers, they did not sponsor this article.
Related Topics
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








