The US is already a monster cannabis market – and it’s not even legal there yet
Health & Biotech
Health & Biotech
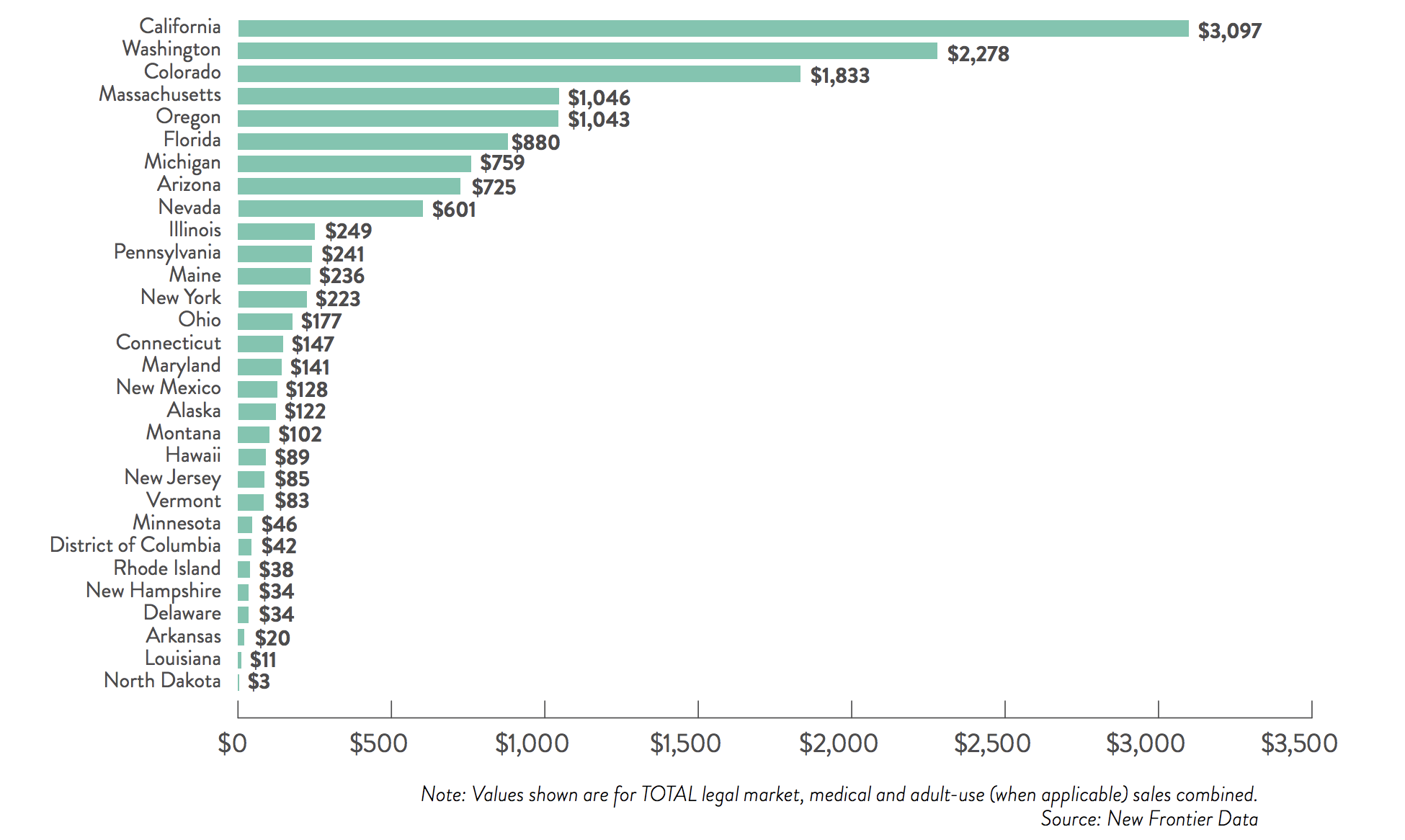
The total US cannabis market is forecast to be worth $US14.5 billion — about half of the global market — across 39 States within 18 months.
Some 2 million Americans are now using medical cannabis to treat serious disease, and two thirds of those say it’s for chronic pain, according to a report by cannabis analyst New Frontier Data.
The report expects the combined recreational and medical markets to grow by 13.7 per cent a year until 2025.
The US is still cut off from global markets because cannabis is technically illegal at a federal level. But Australian companies like Elixinol Global (ASX:EXL) and several corporate advisors spoken to by Stockhead are keeping a close eye on the US with some indications that President Trump might support legalisation.
The US is already a heavyweight in the market. By last year it was already bigger than Canada, generating the equivalent of $11.3 billion in sales compared to $5.9 billion by their northern neighbour.
That could benefit some two dozen ASX-listed stocks with exposure to cannabis. Click here for a list of ASX cannabis stocks.
Right now recreational marijuana is legal in eight US States and in Washington D.C. while medical cannabis is allowed in another 22 States. Nine more are expected to legalise one or the other by 2020.
Biotech bonanza
With the FDA approving the first drug earlier this year, Epidiolex, the US is becoming much more interesting for Australian biotechs, such as Medlab Clinical (ASX:MDC) and the ASX’s newest pot stock Rhinomed (ASX:RNO).
“Sales of Epidiolex will effectively open the pharmaceutical CBD [cannabidiol] market, and the Hemp Business Journal… forecasts the drug to generate between $US15 million and $US30 million in sales through the end of 2018, with $US180 million in sales projected for 2019,” the report said.
With the prospect of lighter regulations on cannabis-based drugs — the Drug Enforcement Administration has to reschedule Epidiolex in light of the FDA approval — pharmaceutical companies are looking anew at the sector.
AbbVie has an FDA-approved, cannabis-based drug on the market called Marinol to alleviate nausea and increase appetite for chemotherapy and AIDS patients.
Cara Therapeutics doing preclinical research on a cannabinoid receptor to treat chronic pain.
Insys Therapeutics is developing a synthetic cannabis drug to treat childhood epilepsy and spray technology to deliver it.
Valeant Pharmaceuticals has made a capsule with synthetic cannabinoids that mimic the effects of THC.
And Zynerba Pharmaceuticals is working on a THC patch for peripheral neuropathic pain, and a CBD-based gel to treat epilepsy in adults with focal seizures, osteoarthritis, developmental and epileptic encephalopathies, and Fragile X syndrome.
The opioid crisis in the US is also spurring States to turn to cannabis.
Maine, Pennsylvania, and New Mexico are actively canvassing drug companies and changing regulations to swap opioids for cannabis.