Researchers say ImpediMed’s device works; shares recover 14pc
Health & Biotech
Early data from ImpediMed’s latest trial shows that its technology is “very sensitive” when it comes to finding small amounts of fluid after breast cancer treatments.
The trial was testing for lymphoedema, or swelling caused by fluids. Women who have had surgery or radiotherapy for breast cancer are particularly susceptible.
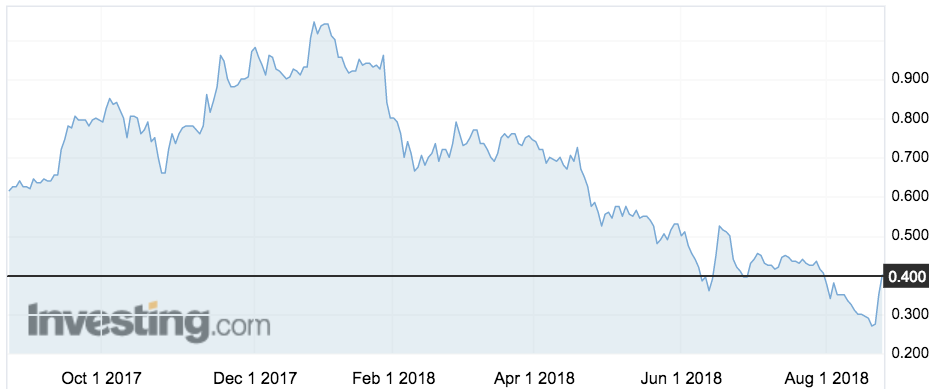
ImpediMed’s (ASX:IPD) shares popped 14 per cent to 40c after the news.
ImpediMed had undergone a rough six months following the release of the preliminary data from the trial, known as “PREVENT”.
The shares fell 75 per cent from a one-year high of $1.07 in January to a low of 26c earlier this month.
The trial started in 2014 to find out if sub-clinical detection of increasing extracellular fluid — that is, before it’s bad enough to show symptoms — via the ImpediMed bioimpedance spectroscopy device L-Dex and early treatment could reduce the rate of progression to chronic lymphoedema.
Bioimpedance spectroscopy is a way to measure fluids in the body.
The patient recruitment finished in December and the trial still has another three years of follow-up to go. The current data is from the first 280 of 1100 patients.
As of January 2018, 109 patients in the L-Dex group had early intervention for lymphoedema, compared to 68 who were being measured with tape measure only.
The final reading of the results says the device is “very sensitive in the assessment of sub-clinical lymphoedema in patients with a history of breast cancer”.
It recommends using the device every three months after surgery for up to a year.
Stockhead is seeking comment from Impedimed.