Opyl’s TrialKey achieves major milestone with potential to improve clinical trial success rates

Opyl has reached a major milestone with its latest software solution TrialKey. Image: Getty
Opyl has reached a major milestone with its latest software solution TrialKey that offers enormous commercial potential for the company but could also be a game-changer in clinical trials globally.
Medtech Opyl (ASX:OPL) has announced that its clinical trial prediction and protocol design software solution TrialKey has achieved a key milestone which has the potential to reduce trial failure rates globally.
TrialKey is a world-first approach using artificial intelligence (AI) to design and optimise clinical trial protocols to overcome the trial outcome data gap.
TrialKey has traced global clinical trials to their outcomes with 92% accuracy, reducing bias in the predictive model.
The milestone is significant as only ~13% of the 431,000 trials registered on the US government website clinicaltrials.gov have posted their results to the site and no trials are linked over time as they progress between clinical trial phases.
The huge information gap is a major problem for the medical research sector as no one benefits from past learnings and often repeat protocol (trial plan) design mistakes made in previous trials.
The information asymmetry contributes to high trial failure rates and the unnecessary waste of time and money on research destined to fail.
The scalable TrialKey Software-as-a-Service (SaaS) solution overcomes this issue by:
- Using machine learning/AI and natural language processing (NLP) to link trials across multiple global databases and sources
- Reducing failure bias and thereby improving predictive and protocol design accuracy and value of the software.
World-first has huge potential
The completion of TrialKey’s first major development milestone was funded under the federal government’s Innovation Connections grant and was done in partnership with RMIT University’s School of Computational Sciences.
Opyl CEO Michelle Gallaher said Opyl and RMIT have together addressed a major barrier in being able to use public datasets in global trial registries to develop a software solution.
“TrialKey can accurately trace and link a clinical trial across phases in minutes when it would typically take five man-hours to complete the same task manually,” she said.
“I am confident that we are close to developing a powerful and valuable offering that can greatly improve the design and outcomes of clinical trials.”
Extensive TrialKey Validation process
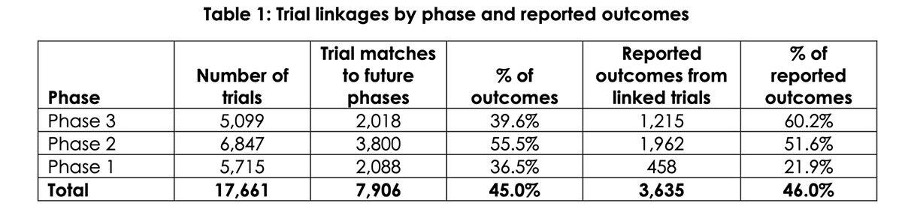
Using a subset of 17,661 Phase 1, 2 and 3 trials sourced from a public registry, the Opyl team, working with RMIT’s Dr Antonio Jimeno Yepes and Professor Karin Vespoor was able to estimate linkages to future phases for the same trial 46% of the time.
For example, table 1 shows that of the 6,847 Phase 2 trials conducted, Opyl was able to find 3,800 Phase 3 trials that link to a Phase 2 study (55%).
The sub-set of trials was based on trials conducted by a selection of publicly listed companies worldwide since 1990.
For a linkage to exist, a trial must have met one of the stated primary objectives in the previous phase.
Although data on the percentage of trials that pass each phase is hard to come by, Opyl considers an overall linkage rate of 45% to be a high match rate based on available estimates of trial success rates by phase 2.
After incorporating linkage estimates, data showed that just 46% of successful trials have reported outcomes, highlighting the lack of overall reporting and poor quality of global datasets within registries. That is, more than 50% of trial outcomes were not reported. No reported outcome is typically considered a ‘fail’, due to the lack of linkage to a follow on trial.
Saving time and money
The linking of clinical trial outcomes across phases leading into an accurate software trial design solution will prove to be an invaluable tool for researchers globally from big pharma to emerging biotech and medtech companies.
With the rising costs of medical research and clinical trials that translates into the higher cost of new drugs and devices, TrialKey aims to reduce failure rates and therefore the waste of valuable financial and clinical resources, which it is hoped will deliver more affordable healthcare.
Dean at RMIT’s School of Computing Technologies Professor Vespoor said the university was excited to work closely with Opyl to help drive innovation and digital transformation in clinical trial design and efficiencies.
“The clinical trials literature is an incredibly rich source of information, not only about the efficacy of new treatments, but also of the factors that contribute to the success of the trials themselves,” Vespoor said.
TrialKey expands Opyl product offering
TrialKey is Opyl’s second clinical trial efficiency solution with its flagship social media-based clinical trial recruitment offering, Opin.ai which was launched in May 2020 and now successfully recruiting patients to studies across ASAP and South America, and in languages other than English.
Opin is gaining strong market traction with global biopharma, contract research organisations (CRO), publicly funded researchers and SME biotechs, successfully using the platform and accompanying service to identify and engage with patients, recruiting for trials and studies around the world.
The company said the two platforms present independent but linked revenue generation and partnering opportunities for the company.
This article was developed in collaboration with Opyl, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








