Neurotech’s Rett Syndrome trial shows improvement in extension stage

The extension stage of NTI’s Phase 1/2 Rett Syndrome trial is looking promising. Pic: Getty Images.
- Neurotech reports positive results from extension phase of Phase 1/2 trial of NTI164 to treat Rett Syndrome
- NTI164 is first broad-spectrum cannabinoid drug therapy to show significant clinical improvement in Rett Syndrome patients
- All 14 female paediatric patients showed significant clinical improvement at 20 weeks
Special Report: Neurotech International has reported further positive clinical efficacy and safety results for all 14 girls who have completed 20 weeks of daily oral treatment with NTI164. This falls under its one-year extension period of a Phase 1/2 clinical trial investigating broad spectrum cannabinoid drug therapy in Rett Syndrome.
Neurotech (ASX:NTI) says patients under the extension phase showed continued significant improvement at 20 weeks when assessed by clinicians using the gold standard Clinical Global Impression-Improvement (CGI-I).
CGI-I measures how much the patient’s illness has improved or worsened relative to a baseline state at the start of NTI164 treatment and ranges from 1 – very much improved, to 7 – very much worse. A decrease in CGI-I score indicates improvement.
Based on the CGI-I primary endpoint at 12 weeks, NTI says it has four core domains of interest including:
- Communication skills
- Mental alertness
- Socialisation/eye contact
- Anxiety
NTI says there was a marked improvement in CGI-I in the four core domains at 20 weeks versus baseline.
Overall, the 20-week data showed an improvement of 33% versus baseline, compared to 23% improvement at 12 weeks.
Between 12 to 20 weeks, NTI says the data showed there was an additional CGI-I improvement of -0.4, representing a significant, additional improvement of 13%, which continues the trajectory of clinical improvement overall.
NTI says the four core domains will likely form the basis of the CGI-I measures required for a registration-directed Phase 3 clinical trial of NTI164 versus placebo.
100% of patients show improvement at 20 weeks
At 20 weeks, 100% of patients showed improvement, compared to 93% at 12 weeks with 57% very much or much improved versus 36% at week 12.
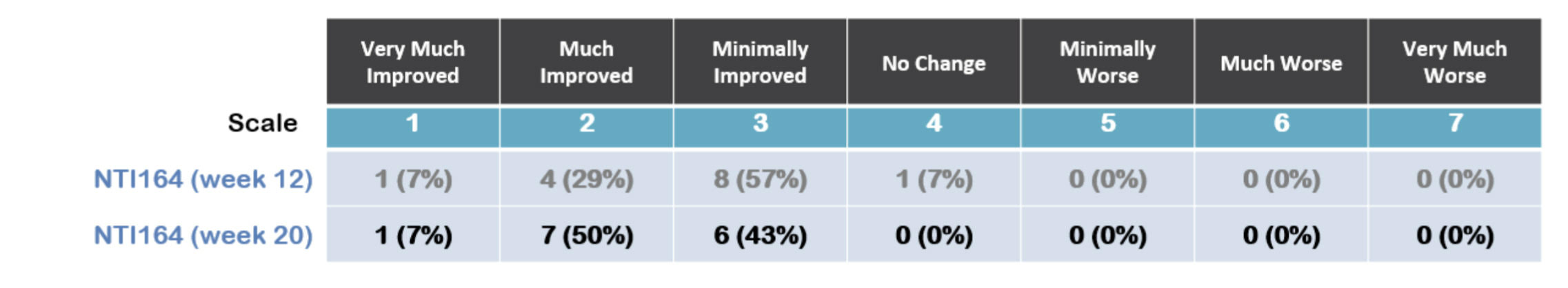
Source: NTI
The individual measures of CGI-I in the four core components measures at 20 weeks all showed continued improvement from 12 weeks.
At 20 weeks, patients receiving NTI164 showed a clinically meaningful 24% improvement in total Rett Syndrome Behavioural Questionnaire (RSBQ) versus baseline.
NTI says there was no further improvement in RSBQ versus baseline at 20 weeks, remaining relatively stable.
NTI says at the trial start the average RSBQ total score for patients was 44.6 compared to 31.2 at 12 weeks and 34.1 at 20 weeks.
RSBQ consists of eight domains/subscales that reflect the core features of the disease including:
- General Mood
- Breathing problems
- Hand behaviours
- Repetitive face movements
- Body rocking and expressionless face
- Nighttime behaviours
- Fear/anxiety
- Walking/standing
Positive safety and weight data at week 20
NTI says there were no serious adverse events (or otherwise adverse events) reported between 12 weeks and 20 weeks, and no weight loss.
The mean weight was stable versus 12 weeks and weight recorded at baseline.
At 12 weeks, two patients (14%) experienced nausea/vomiting effects.
This safety profile compares favourably with the only FDA-approved treatment for Rett Syndrome, which is DAYBUE.
Neuren Pharmaceuticals (ASX:NEU) became the market darling of the biotech sector in 2023 after their billion-dollar partner, Nasdaq-listed Acadia Pharmaceuticals, received FDA approval for trofinetide (now marketed as DAYBUE) in Rett Syndrome.
US$2bn market for Rett Syndrome
NTI reported top-line results for the Phase 1/2 Rett Syndrome clinical trial in April with NTI164 being the first broad-spectrum cannabinoid drug therapy to show a statistically significant clinical improvement in Rett Syndrome patients.
In May, NTI reported further positive results showing significant additional benefits in Rett Syndrome girls after 12 weeks of daily oral treatment with NTI164.
Rett Syndrome is a rare genetic neurological and developmental disorder and is almost exclusively the result of a mutation or mutations in the methyl CpG binding protein 2 (MECP2) gene located on the X chromosome, which is required for normal brain development and function.
The syndrome occurs almost exclusively in girls, with an incidence of one in 10,000 female live births with the prevalence of ~15,000 girls and women in the US and 350,000 globally.
Executive director Dr Thomas Duthy says the company is pleased to see trial patients continue to do very well on extended treatment with NTI164.
“Our safety and efficacy to 20 weeks are trending favourably when compared to the current FDA-approved treatment, DAYBUE,” he says.
“Rett Syndrome represents an attractive market opportunity for NTI164, with a potential annual market opportunity of approximately US$2bn.
“We are currently working with our clinical advisors and our newly appointed chief medical officer Professor Ellaway on the design of a registration-directed Phase 3 clinical trial.”
This article was developed in collaboration with Neurotech International, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








