Neurotech reports more positive Phase 1/2 Rett Syndrome clinical trial results

NTI has reported more positive trial results of its broad-spectrum cannabinoid NTI164. Pic: Getty Images.
- Further primary and secondary endpoint analysis of Neurotech’s Phase 1/2 trial has highlighted additional benefits
- NTI164 is the first broad-spectrum cannabinoid drug therapy to show significantly clinical improvement in Rett Syndrome patients
- All 14 female paediatric patients extended to 52 weeks, with ongoing data to be collected and reported
Special Report: Neurotech International has reported further primary and secondary endpoint analysis of its Phase 1/2 trial NTIRTT1 showing significant additional benefits in Rett Syndrome girls after 12 weeks of daily oral treatment with its broad-spectrum cannabinoid drug therapy NTI164.
On April 17, clinical-stage biotech Neurotech International (ASX:NTI) reported top-line results for the Phase 1/2 Rett Syndrome clinical trial with NTI164 being the first broad-spectrum cannabinoid drug therapy to show a statistically significant clinical improvement in Rett Syndrome patients.
Rett Syndrome is a rare genetic neurological and developmental disorder and is almost exclusively the result of a mutation or mutations in the methyl CpG binding protein 2 (MECP2) gene located on the X chromosome, which is required for normal brain development and function.
The syndrome occurs almost exclusively in girls, with an incidence of one in 10,000 female live births with the prevalence of ~15,000 girls and women in the US and 350,000 globally.
The Rett Syndrome market is estimated at more than US$2bn annually.
The NTIRTT1 trial involved 14 female paediatric patients with an average age of 8.8 years and weight of 27.5kg with a Rett Syndrome Behavioural Questionnaire (RSBQ) mean measure of 44.6 and CGI-severity of illness (CGI-S) measure of 4.6.
The trial participants underwent a 12-week daily oral treatment regimen with NTI164/placebo required for primary endpoint analysis.
The Clinical Global Impression –Improvement (CGI-I) at 12 weeks versus baseline on four core Rett-anchors highlighted 93% of patients improved with 36% “very much/much improved”.
The key secondary endpoint of the RSBQ showed a mean difference of -13.4 versus baseline and a 205% improvement from week four to week 12.
All 14 female paediatric patients have been enrolled in the 52-week extension phase of the trial, with ongoing data to be collected and reported.
Improvement in primary endpoint – CGI-I
Clinical Global Impression (CGI) is a physician/observer-rated scale of the clinician’s impression of the global state of an individual and is frequently employed in clinical trials for neuropsychiatric disorders.
CGI-I is a 7-point scale requiring the clinician to assess how much the patient’s illness has improved or worsened relative to a baseline state at the beginning of the intervention and ranges from 1 –“Very Much Improved” to 7 -“Very Much Worse”.
Top-line primary endpoint analysis reported on April 17 showed a statistically significant improvement in CGI-I at 12 weeks versus baseline measures.
NTI says the top-line CGI-I results were based on analysis available on four Rett-specific assessments (anchors) from a total of nine Rett-specific measures.
As it was the first in human study of NTI164 in Rett, NTI examined other anchors (sub-domains) to further understand which showed improvements that could be targeted in registration-directed trials.
NTI says in late-stage clinical trials typically 2-3 sub-domains are examined for CGI-I.
With the complete data set now available for analysis, NTI reports further improvement in CGI-I versus baseline.
Of the 14 Rett Syndrome patients as measured by CGI-I across all nine Rett-specific measures, 50% of patients showed improvement at 12 weeks with NTI164.
NTI says 50% were minimally improved, 43% showed no change and one patient (7%) was minimally worse.

Image supplied by NTI
Strong improvements in core anchors
NTI has undertaken an analysis of those specific sub-domains cited by doctors and caregivers as important and where NTI164 showed strong improvements.
The drug has shown statistically significant improvements across communication skills, mental alertness, socialisation/eye contact and anxiety, which will likely form the basis of CGI-I measures for registration-directed studies.
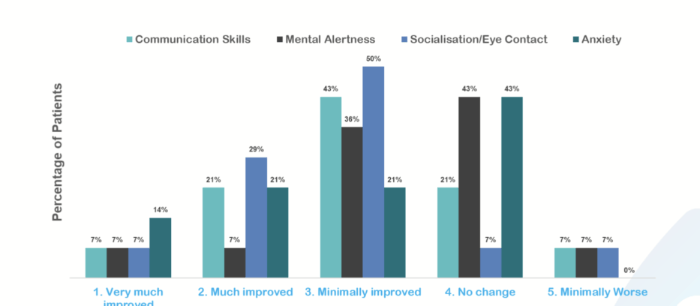
Image supplied by NTI
Secondary Endpoint – RSBQ
NTI says RSBQ consists of 45 items, rated as 0 = ‘not true’, 1 = ‘somewhat or sometimes true’ or 2 = ‘very true’, that can be grouped into eight symptom domain subscales graded on a scale of 0–90 (maximum severity).
The company says eight domains/subscales that reflect the core features of Rett were examined.
The trial showed patients receiving NTI164 had a 205% improvement in their mean baseline change from week 4 (-4.4) to week 12 (-13.4).
Overall, NTI says a clinically meaningful 30% decrease in the patients’ mean RSBQ total score at 12 weeks was seen, which was strongly statistically significant.
At start of the trial the average RSBQ total score for the patients was 44.6 compared to 31.2 at 12 weeks.
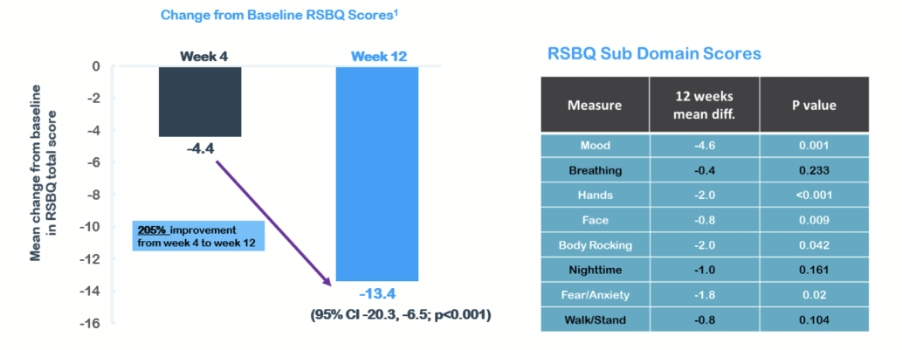
Image supplied by NTI
Another key secondary endpoint – CGI-S – reflects a clinician’s impression of severity of illness on a 7-point scale ranging from 1=not at all to 7=among the most extremely ill. It also showed a statistically significant improvement.
The study also met other key secondary endpoints including Impact of Childhood Neurologic Disability Scale versus baseline and Rett Syndrome Caregiver Burden Inventory.
A single adverse safety event
NTI says one serious adverse event was reported during the 12 week treatment period, with development of urticaria (hives ) in one patient.
The company says Urticaria has not been previously observed in any previous and ongoing studies of NTI164.
Zero patients reported diarrhoea, while nausea/vomiting occurred in two patients. The mean weight of patients was largely unchanged at 12 weeks (0.3 kg gain) versus baseline, indicating overall no weight loss during the treatment period.
None of the adverse events required any additional medications. Measurements pertaining to kidney and liver function along with blood chemistries and vital signs were normal over the 12 weeks.
NTI says NTI164 exhibits an excellent safety profile, with minimal patient-specific side-effects in Rett Syndrome patients.
Promising results
Principal trial investigator and senior staff specialist at The Children’s Hospital at Westmead, Sydney’s Children’s Hospital Network Associate Professor Carolyn Ellaway, is pleased with the trial results.
“The NTIRTT1 clinical trial is the first time a broad-spectrum cannabinoid drug therapy (NTI164) has demonstrated significant patient improvements in Rett Syndrome using validated clinical measures including CGI-I and RSBQ,” she says.
“Our data is very encouraging as we have observed clinically meaningful improvements in those symptoms repeatedly deemed as most important for treating clinicians, caregivers, and patients; notably communication, hand behaviours, anxiety/mood, and quality of life.
“These benefits have not compromised patient safety, with NTI164 displaying an excellent safety profile over the 12 weeks of the trial.”
Rett Syndrome is the third neurological disorder in children where NTI164 has shown a statistically significant clinical benefit, alongside autism and PANDAS/PANS.
“This fulsome data set reported today represents a very rapid translation of our strong conviction on the anti-neuroinflammatory and neuroprotective effects of NTI164 applied to a third paediatric neurological population, Rett Syndrome, where safe and effective therapies are urgently needed,” NTI executive director Dr Thomas Duthy says.
“The level of improvement we have observed in these girls after 12 weeks of treatment is remarkable in the context of the excellent safety profile of NTI164 where just a single serious event was recorded and adverse events were minor and manageable relative to the observed standard therapy in the United States,” he says.
“We look forward to updating the market on these girls’ progress as they progress through the 52 week extension under A/Prof Ellaway’s supervision and presentation of these results at the upcoming 9th World Rett Congress, to be held in Australia later this year.”
A caregiver of a patient in the NTIRTT1 trial said: “She seems much more in tune to what’s going on around her, e.g. patting the dog (has never done this before).”
Another caregiver said: “She uses eye pointing, sometimes brings food/drink to an adult –this has not happened before.”
This article was developed in collaboration with Neurotech International, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








