Green whistle maker Med Dev is living the China dream with a new $46m deal

Pic: REB Images / Tetra images via Getty Images
Medical Developments International is set to triple its annual revenue take with a single deal to sell its non-opioid ‘green whistle’ painkiller into China, Vietnam and Thailand.
For those not familiar, the Penthrox green whistle is a non-opioid pain relief inhaler commonly used in trauma settings for self-administration.
The pharmaceuticals company (ASX:MVP) has signed a deal worth up to $45.8m with Japanese business Daiichi Sankyo to sell Penthrox.
That figure is made up of a $21.1m upfront payment and the rest coming as sales-based milestone payments.
To put that in perspective, in the 2018 financial year Medical Developments made $17.9m in revenue and $243,000 in revenue.
The company’s stock jumped 10 per cent on Monday morning to $5.38.
Chairman David Williams says the vast majority of sales are expected to come from China.
He told Stockhead they had been approached by several big Chinese pharmaceuticals companies because there was a view the country has been under serviced in terms of pain management.
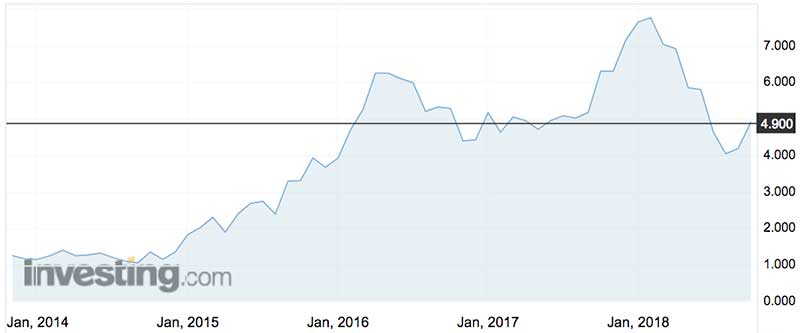
The company started selling the ‘green whistle’ painkiller in 2014, but sales ticked downwards in 2018 from $11m back down to $10m.
Interest in non-opioid based painkillers has taken off after the US opioid crisis began make headlines, but drugs like Penthrox must compete with sexier alternatives derived from cannabis.
In the US, some States are looking to make it possible for patients to the swap opioid drugs they’ve been prescribed for cannabis-based alternatives.
Penthrox is undergoing Investigational New Drug talks with the US FDA now.
Penthrox is currently sold in at least 10 countries, but the only country in Asia is Singapore.
A Chinese hold up
There is a possible sticking point, however.
The two companies must still get Penthrox approved for sale in China, Thailand and Vietnam.
The Australian company will pay for the approval process up to $10m in China, and as a result own any intellectual property gleaned during that process. It is not footing the bill for approvals for the other two countries.
Mr Williams expects the approval process will take around two years and won’t cost as much as the sum they’ve committed.
“Acute pain in trauma and minor surgical procedures is undertreated in the Chinese healthcare system,” said CEO John Sharman.
“MVP believes there is an important place for Penthrox in pain management in China. Penthrox, with its fact acting, non-addictive, non-opioid characteristics will be an important tool for pain management in the Chinese, Vietnamese and Thai markets.”
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








