US roadblocks hurt Aussie green whistle maker Medical Developments

Pic: REB Images / Tetra images via Getty Images
The Penthrox green whistle is an Aussie marvel of pain relief that has been available in this country for the best part of 30 years.
But its global roll-out has just ground to a halt, with US agency the Food and Drugs Administration telling Penthrox owner Medical Developments (ASX:MVP) to suspend work aimed at getting access to the US market.
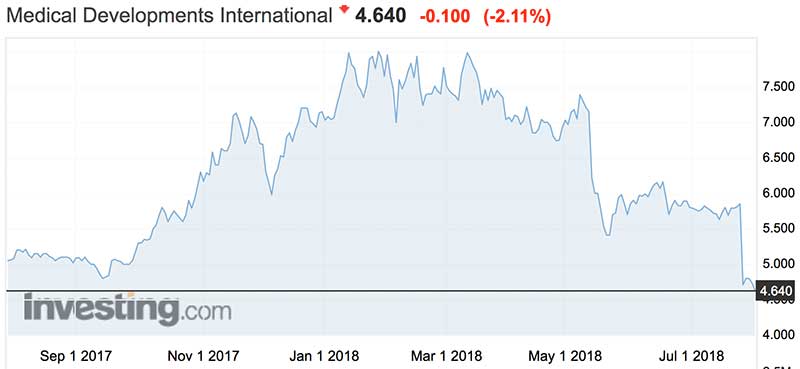
Rising optimism for the growth prospects of the company saw the shares pushed to all-time highs of $7.55 at the start of the year, valuing the company at around $450 million.
But that’s a far cry from the valuation now of $280 million (see graph below).
Investor interest was so strong that last October the chairman David Williams sold 4.35 million shares in the company at $5.40 pocketing $23.5 million.
He followed that up in March, off-loading another 5 million shares at $7.50, for a tidy $37.5 million.
Following the sales, he is still the largest investor, with around 15 per cent of the company.
Unsurprisingly, the roadblock in getting into the US saw the shares dumped, falling back to trade at around $4.60 this week — back at 12-month lows.
To win over the FDA, Medical Developments had set aside $10 million to carry out the necessary research.
Initial animal studies have been done, and it is likely some human trials, too, will be needed.
The additional research work to get into the US mirrors work undertaken to begin selling into other markets.
Regulatory risk is front-and-centre for most medical device outfits, since they have to prove their benefit to win access to markets — along with winning access to funding from health insurers.
One local biotech which has suffered at the hands of regulators in Europe and the US is Clinuvel (ASX:CUV) which has product for treating severe cases of skin pigmentation.
Even though it has been approved for sale in Europe, the UK has refused to provide reimbursement.
In the US, Clinuvel has been working with the FDA for more than a decade to gain access, and has only recently filed its formal application to gain access.
For Medical Developments, over the past few years, the Penthrox green whistle has been approved for sale in the UK, most of Europe, Mexico and Canada.
Revenues are now running at close to $20 million a year — the company is profitable and readily able to fund its development demands.
First used as an anaesthetic, Penthrox was withdrawn in the 1970s for causing liver and kidney damage.
It is now being used in different volumes via an inhaler and has significant advantages in providing quick pain relief to opioid or narcotic drugs.
Unusually, even though the drug was withdrawn in the US at that time, it has not been deregistered.
Still, additional research will need to be undertaken before it is likely to be considered for sale.
Buy, hold or sell?
Analysts are sanguine on the US hurdle.
Bell Potter retains its ‘buy’ recommendation for the shares with a 12-month price target of $7.34. Moelis has a ‘hold’ recommendation with a $5.32 valuation.
Medical Developments expects to hear from the FDA within the next two months, with details of its concerns and any additional research demands to be made clear at that time.
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
Usually, the FDA will either approve the application which would permit clinical trials to get underway or put the application to one side as further information is sought.
Given the deep seated problem with opioid abuse in the US, one school of thought is that the FDA is likely to be cautious in approving Penthrox given the additional public scrutiny it is under.
Even so, the opioid epidemic is seen paving the way for the use of the product.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








