GI Dynamics wants to treat Indian diabetes patients with a gut device

Pic: REB Images / Tetra images via Getty Images
Medical devices company GI Dynamics will kick off a clinical trial in India for a device that treats diabetes and obesity from inside the body.
GI Dynamics (ASX:GID) makes EndoBarrier, an ‘endoscopically delivered’ implant in the intestine that regulates hormones in a similar way to a gastric bypass.
It says for many patients, it reduces blood sugar, weight, and the need for insulin and other medications.
Endoscopy is the practice of using a tube with a camera lens to inspect, or in this case deliver a device, inside the body without performing major surgery.
The company told investors this morning it had entered into an agreement with Apollo Sugar, an Indian diabetes and endocrine healthcare service provider, to conduct safety and efficacy clinical trials.
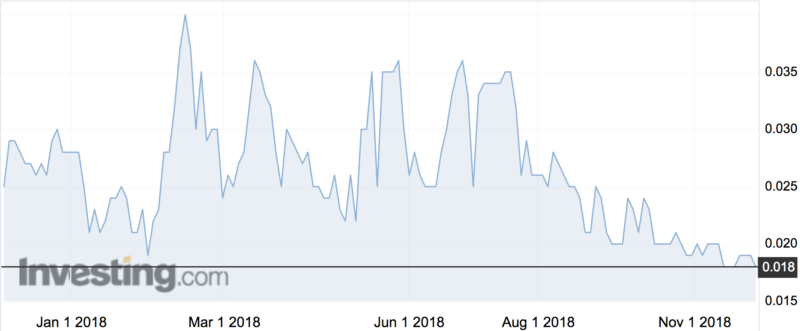
Shares however dipped ever so slightly, down 5pc to 1.8c.
The randomised trial will cover 100 patients, with 75 receiving the EndoBarrier implant and 25 control patients.
It’s expected to begin in the first half of next year and finish by the end of September, if GI Dynamics can raise some extra money, the company said.
“We look forward to working closely with the clinical team at Apollo Sugar to first study the safety and efficacy of EndoBarrier in the region and then supply EndoBarrier to appropriate patients within the region,” GI chief Scott Schorer said.
Gagan Bhalla, chief of Apollo Sugar, said EndoBarrier could provide a “novel and powerful clinical tool” for Indian clinicians.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.







