Exopharm identifies two new lead programs as exosomes gain prominence in gene therapy

Exopharm’s technology has put the company in a leadership-position. Image: Getty
Exopharm’s technology has put the company in a leadership-position in both naïve exosomes and engineered exosomes as it progresses to a product-first company.
It’s been a challenging yet productive two years for the Exopharm (ASX:EX1) team.
The company has made significant progress over this period and now owns a ‘tool chest’ of 7 important exosome manufacturing technologies, as well as well identifying two new in-house GM (genetic medicine) product programs.
At the centre of Exopharm’s is its proprietary manufacturing technologies, which enable the company to develop its own exosome-medicine products and harness their unique drug-delivery capabilities.
Preliminary results from a recently completed study detected no immunogenicity or toxicity of exosomes manufactured by Exopharm, which means they are suitable for clinical pursuit.
The study also helped to validate Exopharm’s exosomes as a safe drug-delivery ‘chassis’ – meaning they could be used as safe ‘delivery vehicles’ inside a person’s body to deliver drug doses at high frequencies.
Separate earlier clinical trials with the company’ Plexaris product also demonstrated safety and efficacy in wound healing using Exopharm-produced exosomes.
These results have now put Exopharm in a leadership-position in both naïve exosomes and engineered exosomes.
Exopharm as a product-company foremost
Exosomes are naturally occurring nano-vesicles that are secreted in huge numbers by cells throughout the human body, and are a natural and powerful way that cells communicate and coordinate.
Within the last 7 years, researchers have envisaged using exosomes as delivery vehicles inside a person’s body – like programmable, targeted and safe nano-sized drones.
Drugs that had delivery problems, like mRNA, DNA and some small molecules, could be packaged into exosomes at a bioprocessing facility to produce modern medicines which were safer, more tolerable, and more specific to the target tissue/destination than their cargo alone.
Over the last few years, Exopharm had worked on developing the technology to manufacture these exosomes, and that led the market to value it as a ‘platform technology’ company.
But now, the technology has developed significantly and Exopharm believes that market perception is outdated.
Exopharm emphasised that it is now a product-first company after announcing two GM programs under development.
Two new lead programs
The Exopharm team has assessed hundreds of potential exosome products over the past 15 months using strict assessment criteria and detailed commercial scoping.
From that work now comes two new programs – with several potential product variants in each program.
Cystic Fibrosis (CF) program
The first lead program is treating Cystic Fibrosis (CF) using exosome-based additive CFTR mRNA gene therapy and nebuliser delivery to lungs.
CF is the most common autosomal recessive disease with >100,000 CF patients worldwide (around 40,000 in US) and the median age of death is 32 years. Lung disorder is the main cause of morbidity and mortality.
There is no cure for cystic fibrosis, but treatment can ease symptoms, reduce complications and improve quality of life.
Current treatment options include antibiotics, anti-inflammatory medications, mucus-thinners, bronchodilators, oral pancreatic enzymes and CFTR modulators.
Exopharm however believes that additive gene therapy is ideal for CF compared to some other genetic diseases.
The Exo-RNA approach, which is gene therapy delivered via exosomes, has several advantages which includes avoiding the use of viral vectors.
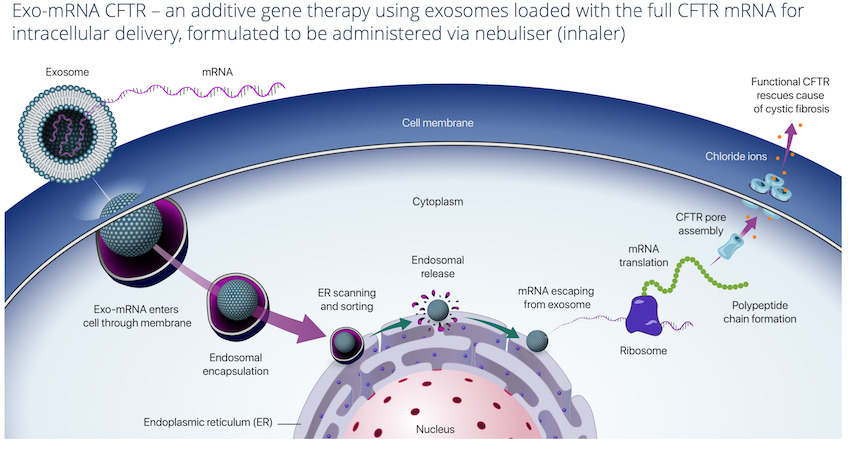
The Exo-RNA gene therapy approach also poses no risk of potential insertion into the host genome as the exosomes loaded with CFTR mRNA are formulated to be administered via a nebuliser (inhaler).
This approach is expected to be more efficacious and cost-effective, with no direct competitors identified.
The CF market is predicted to reach US$31 billion by 2027 with 24% compound annual growth rate (CAGR), up from US$5 billion in 2019.
The Elastin program
Elastin is a natural molecule in our bodies produced from the ELN gene. Elastin imparts elasticity in various tissues such as skin, lungs, blood vessels and fascia.
With age and exposure to smoke, levels of elastin decrease and medical and aesthetic issues arise from that elastic deficiency.
An additive gene therapy using ELN mRNA would produce additional Elastin in the body and could bring the elastic tissue structure back to normal.
Exopharm has identified several main product opportunities in this program including Chronic obstructive pulmonary disease (COPD) such as pulmonary emphysema and chronic bronchitis.
Destruction of elastin or abnormalities in elastic fibre assembly are major factors in emphysema and COPD, and an estimated 3.1 million Americans have been diagnosed with emphysema and around 11.2 million people in the country have COPD.
Tobacco use is the number one factor in the progression of COPD, along with pollution, infections and genetic factors.
Other potential treatments in this program also include skin conditions and aesthetic dermatology.
Exopharm will also explore hypertension and arterial stiffness, scar prevention, and photoaging in its Elastin program.
An emerging opportunity
Exopharm says these two programs have been selected after considering their potential future commercial value, and as a way to showcase exosomes as a non-viral nanoparticle ‘chassis’ to overcome drug-delivery problems that GMs face.
With these two lead programs, the company believes its strategy and future-direction is clear and promising.
Exopharm believes there is an emerging opportunity to treat many medical problems with Genetic Medicines to use exosomes as the drug-delivery chassis for potentially hundreds of new products.
Exopharm can also profit from this opportunity by making its exosome technologies available to others on commercial terms.
The company can also generate revenue by developing a small number of its own products in order to build shareholder-value along the way.
This article was developed in collaboration with Exopharm, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








