- Argenica receives $2.089 million via R&D Tax Incentive Program to continue developing its ARG-007 lead drug
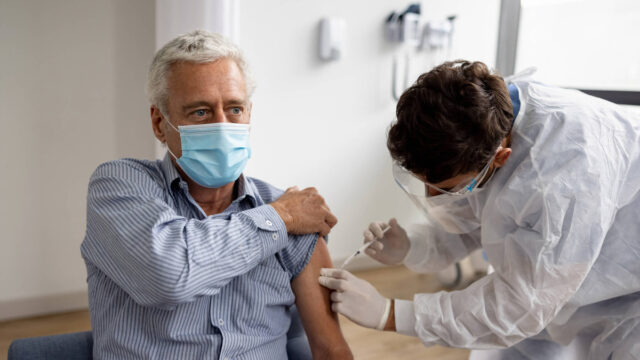
- Funds will be invested in upcoming Phase 2 clinical trial on ischaemic stroke patients
- Hospitals across all major Australian cities confirmed to be participating in the double-blind study
Special Report: Argenica Therapeutics has received a R&D cash rebate of almost $2.1 million, which can be applied to further developing ARG-007, including investing back into the upcoming Phase 2 clinical trial on ischaemic stroke patients.
Perth-based Argenica Therapeutics (ASX: AGN) is developing novel therapeutics to reduce brain tissue death after stroke, other types of brain injury, and neurodegenerative diseases.
Argenica’s lead neuroprotective peptide candidate ARG-007 has already successfully demonstrated improved outcomes in pre-clinical stroke models, traumatic brain injury (TBI) and hypoxic ischaemic encephalopathy (HIE).
The ~$2.1m received from the Australian Government’s R&D Tax Incentive Program will be used to continue development of ARG-007. The company was recently given the green light to undertake the Phase 2 “proof of concept” clinical trial of its lead drug for acute ischaemic stroke patients.
Major hospitals in WA, South Australia, VIC, NSW and QLD have all confirmed their participation in the upcoming trial with site governance due to be completed later this quarter.
Eligible patients will receive either a single intravenous dose of ARG-007 or saline placebo prior to undergoing a thrombectomy procedure. The trial will be completed as a double-blind study.
‘Vital for supporting Australian innovation’
An “advance and overseas” finding has also been approved by AusIndustry, enabling both domestic and overseas expenditure on Argenica’s planned pre-clinical efficacy, non-clinical studies, manufacturing, regulatory activities and Phase 2 clinical trial requirements to be included as eligible R&D expenditure for the next three financial years.
Argenica managing director Dr Liz Dallimore said the R&D Tax Incentive Program was vital for supporting Australian innovation.
“These funds will be applied to further developing the company’s lead neuroprotective peptide candidate, ARG-007, including an upcoming Phase 2 clinical trial in ischaemic stroke patients, as well as continuing to generate pre-clinical data in other neurological conditions, including in TBI, HIE and Alzheimer’s Disease,” she said.
The recently completed Phase 1 clinical trial in healthy human volunteers assessed the safety and tolerability of a single dose of ARG-007
Pre-clinical data has also shown ARG-007 inhibits another of the main abnormal proteins which causes Alzheimer’s Disease.
This article was developed in collaboration with Argenica Therapeutics, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
You might be interested in