Adherium’s focus on US market could pay off big time for its novel asthma device

While inhalers are effective, people are not taking them correctly, says Adherium’s CEO, Paul Mastoridis. Picture Getty
- Asthma affects hundreds of millions of people globally
- While inhalers are effective, people are not taking them correctly
- Adherium addresses this problem, as we reached out to CEO Paul Mastoridis
It’s one of the most common chronic diseases worldwide, affecting more than 300 million sufferers of all ages globally.
In the US alone, this disease costs the American healthcare system US$82 billion a year.
We’re of course talking about asthma – a common chronic respiratory condition that affects the airways, causing them to become inflamed and narrow, leading to symptoms such as wheezing, coughing, shortness of breath, and chest tightness.
Unfortunately there’s really no cure at the moment for both asthma and COPD (chronic obstructive pulmonary disease).
“Patients are going to have the disease for the rest of their lives, so they have to take these inhalers for the rest of their lives,” said Dr Paul Mastoridis, the newly hired CEO of Adherium (ASX:ADR) which is a sub $10m market capped company focusing on this space.
“But along the way, we don’t do a good job of educating patients about why it’s important to take these inhalers every day.
“A lot of people say ‘well I feel good today’, so they forget about taking it.
“But it’s when you feel good that bad things happen, because when you stop taking that medication, all of a sudden you get an asthma attack,” said Mastoridis.
Adherium’s technology solves that problem by attaching sensors to the inhalers to not only remind people to take the medicine, but also telling them whether they’re inhaling it correctly.
How the device works
Called the Hailie Smartinhaler, Adherium says it’s one of the leading, technically advanced Digital Adherence Monitoring Systems in the world.
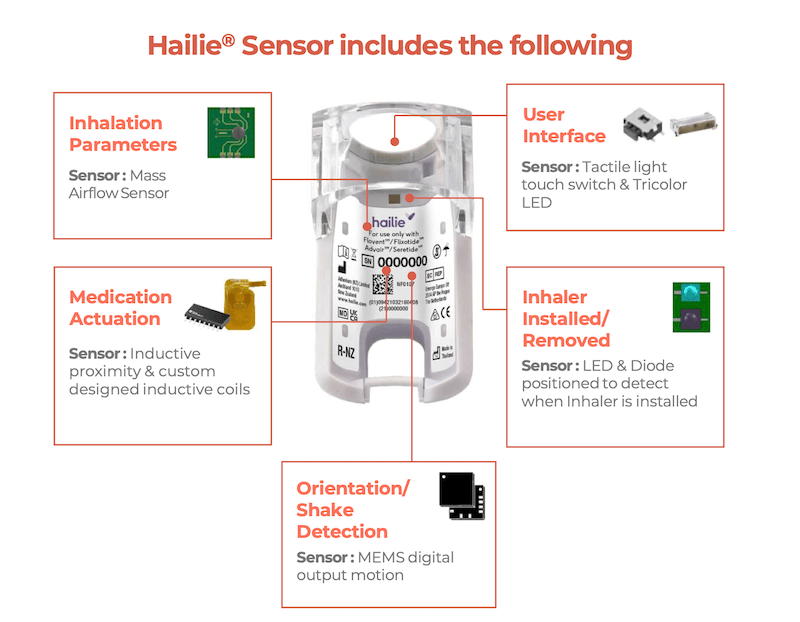
The Hailie device attaches on to the inhaler and is linked to an app (paired via Bluetooth), giving valuable information such as how regular and how well patients are inhaling the medicine to make sure they’re taking it as prescribed.
“We have good evidence showing that patients who do take their medication as prescribed – ie; they’re adherent to their medication or at least 80% adherent – will have less exacerbations, less asthma attacks, less ER visits, less hospitalisations, and subsequently, less deaths,” Mastoridis told Stockhead.
Another problem that patients have is they’re not taking their asthma inhalers correctly, or what’s called ‘poor technique’.
“We have over six sensors built into the Hailie device. The sensors have rotations that will allow you to know if you’re rotating the inhaler correctly.”
The sensors also measure flow rate; in other words, it can tell if you’re actually inhaling the medicine optimally into your lungs.
“Just pressing the button [of the inhaler] doesn’t give you credit for adherence on our platform. You must physically take the medication into your lungs to get credit for adherence,” explained Mastoridis.
“So it’s really a technologically advanced pioneering system that helps patients, and provides lots of information to physicians on how to manage that patient.”
FDA and Artificial Intelligence
Mastoridis says Adherium is the only company out there with the technology that provides this kind of service.
As such, the device recently received clearance from the U.S. Food and Drug Administration (FDA) for use with AstraZeneca’s Airsupra and Breztri inhalation devices.
Airsupra is the first FDA approved rescue medication for asthma patients, while Breztri is a triple combination for COPD patients.
“We’ve been partners with AstraZeneca for a while, as we were involved in some of their clinical trials. But this was the first time AstraZeneca has a rescue medication that’s been approved by the FDA, and it’s the only one of its kind,” said Mastoridis.
“We now have over 90% of the inhalers out there that fit our Hailie device.”
“In total, we have 12 FDA approved devices. So we can now say to the physicians, whatever device or whatever drug you want to give to patients, we have a Hailie device.”
In addition, Mastoridis says that Adherium is essentially moving from a clinical trial company to a data management company.
“The patient gives us consent, and we can use that to collect information such as how well they’re reacting to medication, or what type of patients they are.
“Those data can then be given to health insurers, allergy partners, or the governments who are paying for this.
“More importantly, we can use this data to build an AI (artificial intelligence) system to be able to predict an asthma attack before it happens.
“So there’s a lot of information and value in the data that we have,” says Mastoridis.
Focus on the US market
Over the next two years, the sole focus of Adherium will purely be on the US market.
“The US is where we get reimbursement, so we need to get the model right there. We need to work with allergy partners and SENTA to grow the business,” Mastoridis said.
SENTA (Southern Ear, Nose, Throat and Allergy) is one of the largest, premier specialty allergy and asthma groups in the US. In September 2023, Adherium announced that the Hailie platform would become available to SENTA clinics and patients.
Adherium is also collaborating on a study conducted by US-based Intermountain Health, one of the largest hospital systems in the world.
“We’re doing a study with them to see if our Hailie products can prevent hospital readmissions after 30 days,” says Mastoridis, adding that Intermountain is often cited by US Presidents, and works with the CMS, Medicare, and most other payors in the US medical system.
“CMS is very much interested in that study, because if we can prove that Hailie is able to prevent readmissions, hospitalisations, and ER visits, it will become a standard of care not only for Intermountain, but for all of the US in both asthma and COPD.”
Mastoridis also believes that the Adherium stock is undervalued at the current price of 2c.
“That price is nothing compared to where we’re going and what we’re going to do. Right now is the first time we’re actually getting reimbursement in the US for our services.
“We’re in the biggest pharmaceutical market and in a country where these devices can actually get paid. So I believe now is the time to invest in us,” said Mastoridis.
Other ASX respiratory focused stocks
Respiri is the maker of the wheezo medical device, which is said to be the world-first FDA-approved Class II medical device that analyses breath sounds for wheeze in asthma patients.
The device works with the Respiri app, enabling users to log symptoms and triggers.
Like Adherium, Respiri is also focusing on the US market, and has this week announced a 3-year contract with Hawaii Independent Physician Association (HIPA) – Hawaii’s largest association of independent physicians – to deliver remote patient monitoring (RPM) services to high-risk patients.
Medical Development International (ASX:MVP)
MVP’s flagship product is Penthrox, commonly known as the ‘green whistle’.
MVP has reported positive momentum on the Penthrox entry to the US market, with a meeting with the FDA conducted in October last year.
The company says its priority now is to forge a deeper understanding of US market potential, sales channels, potential partners and opportunities.
ResApp
Worth mentioning here is ResApp, a University of Queensland startup that was acquired by Pfizer in 2022 for $179 million (and now no longer listed on the ASX).
ResApp developed simple and inexpensive smartphone technology that can accurately identify respiratory diseases based on cough analysis.
The technology records a patient’s cough on a smartphone and analyses sounds and simple symptoms, such as a runny nose, to diagnose and measure the severity of a range of chronic and acute diseases.
They include asthma, pneumonia, bronchiolitis, croup and chronic obstructive pulmonary disease.
Share prices today:
The views, information, or opinions expressed in the interview in this article are solely those of the interviewee and do not represent the views of Stockhead.
Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








