Control Bionics (ASX:CBL) launches world’s first autonomous driving wheelchair module
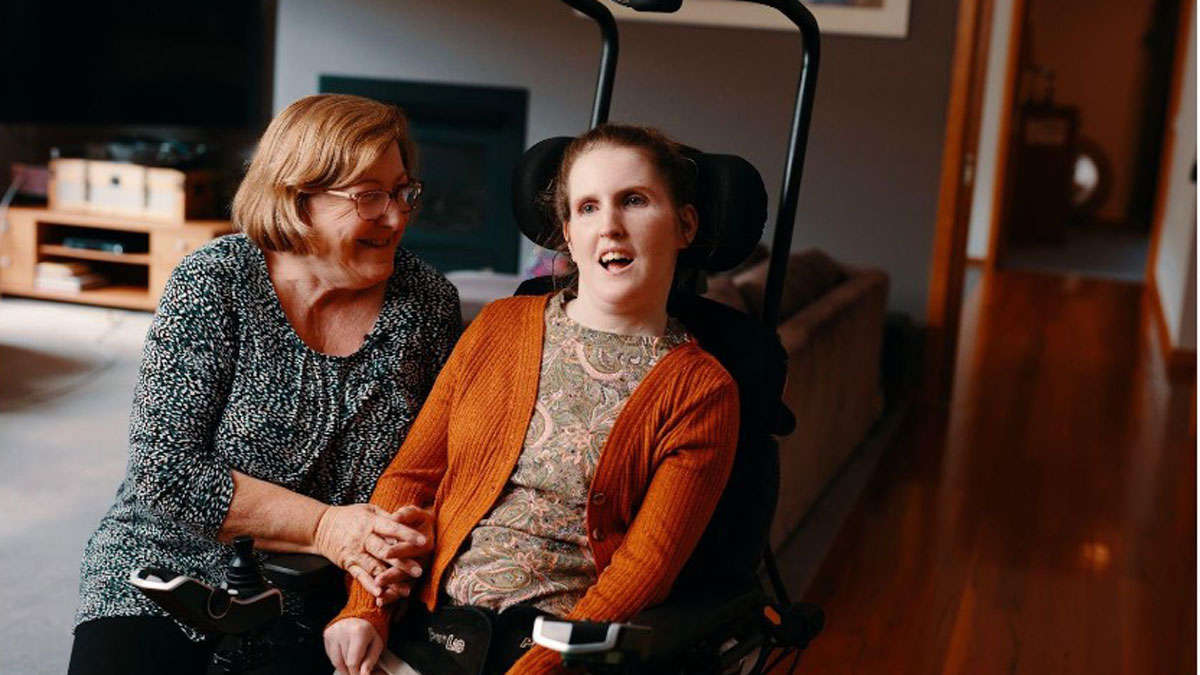
Brodie Shaw tests the DROVE module at her home. She lives with cerebral palsy. Image Supplied.
The Australian medical technology pioneer is expanding its offering to enable those living with severe physical disability to move as well as communicate.
Control Bionics (ASX:CBL) has unveiled the newest offering in its range of world-class technology for the global disability market with the launch of DROVE – the world’s first autonomous driving wheelchair module.
Created in collaboration with Deakin University, the ground-breaking Australian manufactured technology can be retro-fitted to powered wheelchairs and allows users to specific locations within the home – a world first.
Control Bionics is a global leader in electromyography (EMG), capturing and processing bioelectric signals into electronic commands to allow users to do everything they would normally do with a keyboard, mouse, joystick or touchscreen.
Growth trajectory
The DROVE module is the latest application of the company’s innovative NeuroNode technology which has enabled people living with severe disabilities to communicate despite being completely “locked in”.

CEO Jeremy Steele said the launch of DROVE sets the stage of the next phase of growth for the company, building on the vision of founder tech entrepreneur Peter Ford.
“DROVE is a natural stepping stone in the global disability medtech space which is worth around $2 billion,” he said.
“Our NeuroNode technology allows people who are completely locked in to communicate and now DROVE will enable them to safely move around their home. It’s a level of independence they’d never considered possible.”
Steele, a medtech industry veteran, joined Control Bionics in January after a decade leading global cardiac testing service provider CardioScan.
He said DROVE sets Control Bionics up to be a long term player in the developing AI and robotics industries within and outside the med tech space.
“It gives resellers a product they can market with new wheelchairs as well as refit to existing ones,” he said.
“It will leverage key distribution relationships established in the last two years with major companies like Quantum Pride and Numotion.”
The company is expecting the DROVE system to be available under the NDIS and Steele said the hope is that it will give independence to users and take some basic tasks from carers.
“To giving independence, security and control to those living with restricted movement from diseases such as sclerosis, cerebral palsy and motor neurone disease (MND) as well as spinal cord injuries, was the driving reason behind Control Bionics’s inception and DROVE goes a long way toward that,” he said.
Sales strategy
Since its IPO in late 2020, Control Bionics has been focussed on building out a business platform that maximises the long-term sales potential of its technology in Australia, North America and Japan.
The company now has specialised sales teams in these areas and has appointed senior staff to lead critical operation aspects of the business.
In its recently announced half year results, the company said it expected to see solid growth in sales and productivity gains throughout FY23.
Revenue for six months to December 31 was up 23% compared to the same time period the year before.
Sales in North America and Australia rose 24% and 18% respectively. Cash receipts from customers rocketed up 91%.
This article was developed in collaboration with Control Bionics, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








