What’s ahead for stem cell play Cynata after that huge run

Pic: Charnchai / iStock / Getty Images Plus via Getty Images
Stem cell play Cynata Therapeutics has just passed its first day of reckoning, with early results from a phase-one trial of its treatment for graft versus host disease (GvHD).
While there’s a long way to go, the results support Cynata’s core approach to the production of mesenchymal stem cells (MSCs).
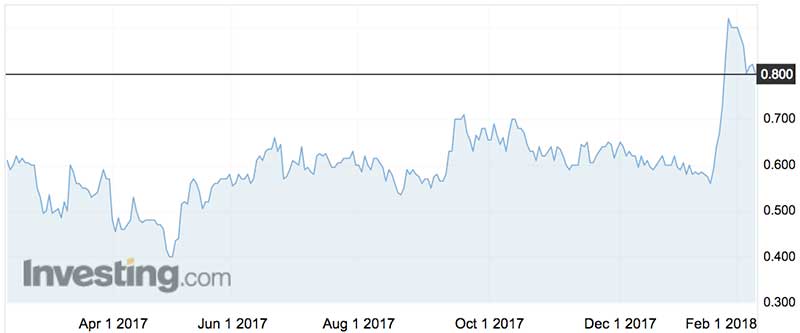
Investors certainly liked it, pushing the shares (ASX:CYP) from 56c to 92c before last week’s overall market ‘bondcano’ stole some of the gains.
Crucially, further positive trial results could prompt partner Fujifilm to exercise an option over the technology, thus delivering a $US3 million upfront payment and milestones worth up to $US60 million and ongoing double-digit royalties.
The key point here is that Fujifilm would also cover the cost of developing the drug further.
Plan B — scrounging for another party — is not so picturesque.
“But we are not against the burden of coming up against this cold,” says ever-optimistic chief executive officer Dr Ross Macdonald.
“We know that MSCs work clinically.”
As an ASX entity, Cynata derived from Eco Quest, which sold green-friendly disposable nappies before getting into the poo. The nappies were then consigned to the smelly garbage bin and Eco Quest relisted as Cynata in October 2013.
The stem cell dilemma
Along with proving the efficacy of the technology to treat specific diseases, the big challenge with stem-cell therapeutics is procuring enough of the raw material in the first place.
In short, Cynata claims to be the only stem cell play in the world that can produce MSCs on a commercial scale without requiring multiple donors.
“We have taken a product that is pretty darned good but said here’s a better way of manufacturing it,” Dr Macdonald says.
To date, the precursor material has been derived either from embryos, or a process called bone marrow aspiration. For bone marrow aspiration multiple donors are required and the coveted MSCs are only present in small amounts.
MSCs are adult stem cells which can be isolated from human and animal sources and can produce more than one kind of specialist cell.
An invasive surgical procedure, bone marrow aspiration sounds painful because it is painful. As for tapping embryos, the practice is controversial because some people see a fertilised embryo as a human in its own right.
Under pressure from the religious right, George W Bush outlawed harvesting spare embryos from in-vitro fertilisation (IVF) procedures, only for Barack Obama to rescind the measure. Trump, meanwhile, is yet to Tweet his official stance.
“While many others strongly disagree with that view, especially within the scientific community, the vociferous opposition from some quarters continues to hamper progress with embryonic stem cell research,” says Cynata co-founder Prof Igor Slukvin.
… and Cynata’s answer
Cynata hopes it has the solution with its patented Cymerus manufacturing process.
The technology is based on induced pluripotent stem cells (IPSCs), from which MSCs are derived and don’t require embryos or bone marrow aspiration.
The “pluripotent” bit means the IPSCs have the ability to develop into any type of adult cell. They can be derived from anywhere in the body — typically skin and blood — and grown in limitless quantities in the lab.
IPSCs derived from the work of Prof Slukvin, from the University of Wisconsin-Madison as well as Japanese research. UWM is a global leader in stem cell research, while Japanese researcher Prof Shinya Yamanaka won a Nobel Prize in 2012 for his work in the area.
- Bookmark this link for small cap breaking news
- Discuss small cap news in our Facebook group
- Follow us on Facebook or Twitter
- Subscribe to our daily newsletter
The MSC sector is certainly active, with 650 trials now taking place including for cardio vascular, lung disease (such as asthma) and strokes.
Just to be clear, Cynata doesn’t intend to be a mere factory that supplies the material to others: it aims to develop drugs, starting with its putative GvHD remedy CYP-001.
An immunological disease, GvHD afflicts bone-marrow recipients and is usually fatal in the case of candidates resistant to steroid treatment. It’s also a key target for Mesoblast, which is running a paediatric trial in the US.
One GvHD drug has already been brought to market: JCR Pharma’s Temcell, licensed from Mesoblast and originally acquired from Osiris. But Dr Macdonald says Fujifilm still believes a new drug would be worth $US300 million in annual sales, which would deliver at least $US30 million in annual royalties to Cynata.
Cynata’s less advanced therapeutic targets include heart attacks, acute respiratory disorder syndrome, glioblastoma and critical limb ischemia.
DSMB says CYP-001 for GvHD is A-OK
On January 22, Cynata proclaimed that the independent data safety monitoring board had recommended that it treat the second cohort of GvHD patients with CYP-001, after all eight patients in the first (lower dose) treatment group showed a partial response to the treatment.
This was measured by an improvement of at least one grade form the baseline on the GvHD scale. One patient subsequently died of pneumonia, but this was deemed not to be trial-related.
A further eight patients will be treated with a higher dose across seven treatment sites here and in the UK, with results expected later this year.
The trial is significant because it is the first time globally any patient has been treated with an IPSC derived MSC product of any description, the patients’ own cells or otherwise.
“A successful outcome will support the application of CYP-001 in many medical and commercially significant targets where therapeutic MSCs have shown promising results,” Dr Macdonald says.
In both groups, the patients haven’t responded to traditional cortico-steroid treatment and are most unwell.
Dr Boreham’s diagnosis:
We’re loathe to say something as hackneyed as the $73 million market cap Cynata is the poor man’s version of the $600 million market cap Mesoblast. But we’ll say it anyway.
Success is far from guaranteed. As the company acknowledges, there’s a lot of time and money going into IPSC research globally and scalable products have already been achieved (including blood, heart, vascular and connective tissue cells).
Cynata is confident that in the next decade several IPSC products will be approved for clinical use and become commercially available.
Like every self-respecting chief executive officer, Dr Macdonald believes the stock is undervalued but at least he offers some solid evidence: in early January Japanese pharma giant Takeda bought Belgian stem-cell developer Tigenix for $US620 million.
“This creates a fear of missing out among all other drug companies including Fujifilm,” he says.
Fujifilm aside, Cynata’s register is oriented to retail investors, with the stock featuring regularly on the Hot Copper tattle site (much to Dr Macdonald’s chagrin). A notable holder is John King, founder of the Storage King empire and a keen investor in speccie techies.
The stock is not well covered. But US research house HC Wainwright & Co (formerly Rodman & Renshaw) takes a stab at a $1.50 a share valuation, “based upon the valuation of the platform on collaboration-based revenue only”.
With cash of $8.8 million Cynata is self-sufficient until 2019, but every time Crucible says that about a biotech the company then passes around the hat for funds.
This column first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner and does not possess a doctorate of any sort – or even the early precursor of a Nobel Prize, as far as he knows.
The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








