Weed Week: Blow to cannabis as Thailand backpedals on weed law; and recent ASX pot stock winners

Blow to cannabis after Thailand backpedaled on recreational weed. Picture Getty
- Blow to cannabis after Thailand backpedaled on recreational weed
- Netherlands set to expand its cannabis decriminalisation to full legality
- Half of Aussies say yes to growing pot at home
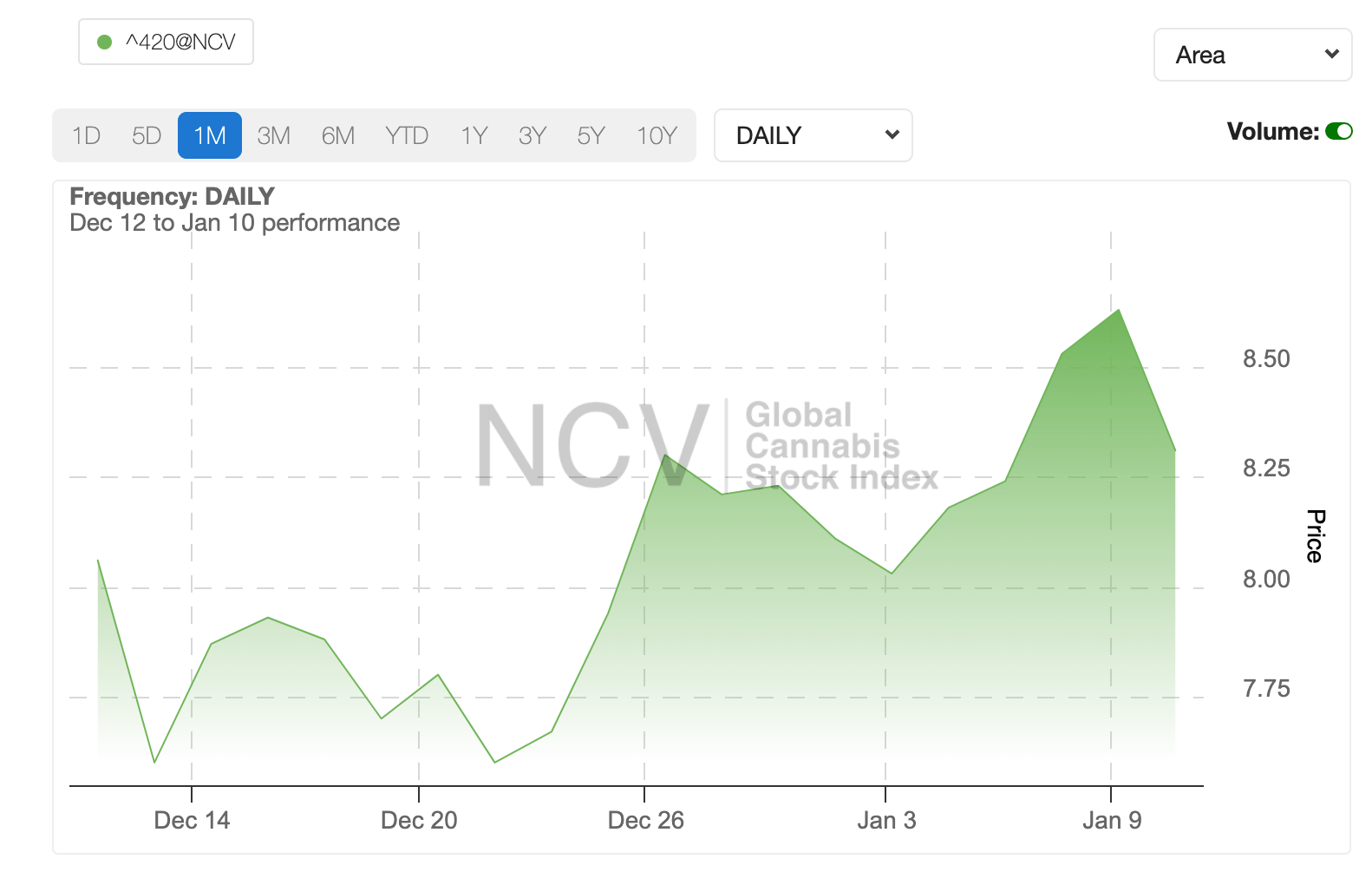
It’s been a good four weeks for global cannabis, with the NCV Global Cannabis Stock Index rising by around 7%.
But in a setback to the global cannabis industry, Thailand has proposed a bill that will ban the recreational use of cannabis and impose hefty penalties on offenders.
Under the draft bill, smoking of marijuana and its use in any form for recreation in Thailand will be banned. The use of cannabis will instead be limited to medical and health purposes.
The country has backpedaled after becoming the first Asian nation to decriminalise cannabis in 2022.
Since then however, the local industry has been operating in a grey area because the decriminalisation happened before Thai lawmakers could agree on how to regulate it.
The move to ban recreational use fulfils the election pledge of new Prime Minister Srettha Thavisin, who’s looking to end the regulatory vacuum that has led to the mushrooming of thousands of cannabis dispensaries all over the country.
Meanwhile in the Netherlands, a pilot program has begun that could expand the country’s tolerance of cannabis to full legality.
Coffee shops in Breda and Tilburg, two cities in the southern part of the country, are now legally allowed to sell pot from registered suppliers.
The trial has been hailed by the Dutch health minister, Ernst Kuipers, as a “historic moment”, and could now pave the way to a full decriminalisation across the country.
A common misconception is that cannabis is already legal in the Netherlands, but in reality, the industry is operating in a grey zone where consuming cannabis is still technically illegal.
“We are well positioned to take the Netherlands back to the forefront of the cannabis industry,” said Ashwin Matai, a Dutch cannabis plant grower.
Half of Aussies give thumbs up to growing pot at home
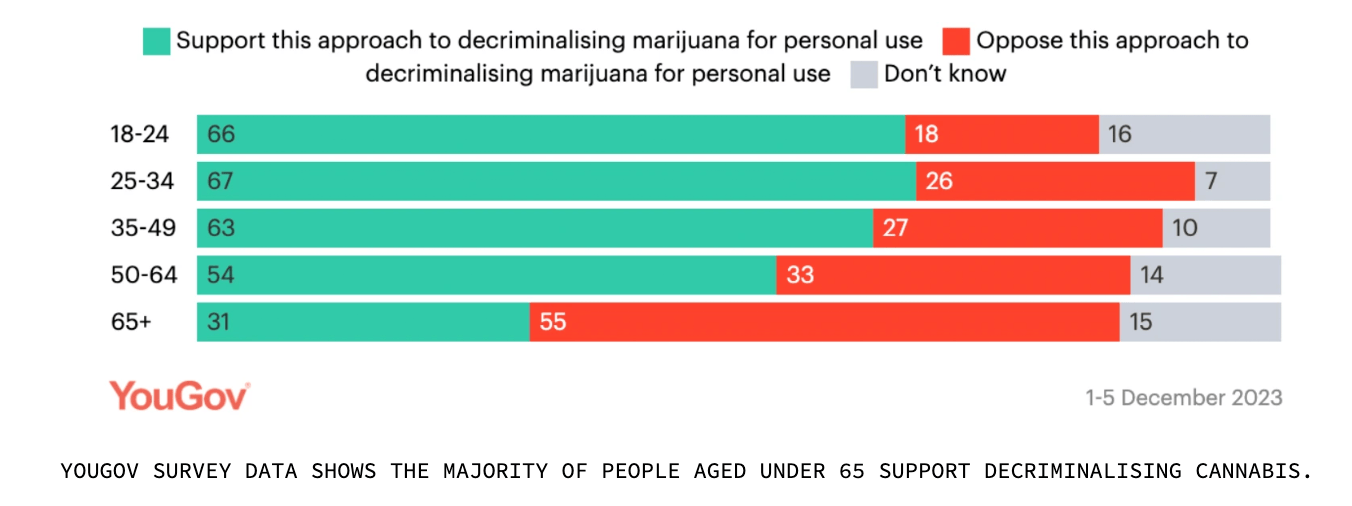
Back home, a new survey found that 50% of Aussies support legalising growing cannabis at home for personal use.
The data was gathered from a survey of 1,555 participants conducted by YouGov in December, which also showed that 31% opposed the idea while 19% were undecided.
The survey also showed that 54% were in favour of decriminalising cannabis.
Australians aged 18-24 were the most in support of the cultivation bill, as well as decriminalisation of cannabis.
YouGov’s director of Polling, Amir Daftari said:
“This survey experiment shows that the majority of Australian voters support both propositions; decriminalisation of cannabis for personal use at 54%, and legalising cannabis for personal use at 50%.
“This support is consistent across states and age groups.”
To ASX Weed Stocks ….
Here’s how the ASX weed stocks have performed, sorted by winners over the past month
| Code | Company | Price | % Week | % Month | % 6-Month | % Year | Market Cap |
|---|---|---|---|---|---|---|---|
| ALA | Arovella Therapeutic | 0.130 | 8.33 | 47.73 | 160.00 | 550.00 | $118,625,155 |
| LV1 | Live Verdure | 0.485 | 29.33 | 46.97 | 432.97 | 185.29 | $56,618,364 |
| NTI | Neurotech Intl | 0.073 | -3.95 | 28.07 | 58.70 | 4.29 | $65,096,964 |
| BOT | Botanix Pharma | 0.173 | -9.21 | 15.00 | 32.69 | 187.50 | $272,164,073 |
| AVE | Avecho Biotech | 0.004 | 33.33 | 14.29 | -33.33 | -69.23 | $9,507,891 |
| EOF | Ecofibre | 0.130 | -10.34 | 13.04 | -27.78 | -49.02 | $49,253,607 |
| DTZ | Dotz Nano | 0.155 | -6.06 | 10.71 | -36.73 | -38.00 | $79,652,157 |
| EMD | Emyria | 0.070 | 27.27 | 7.69 | -51.36 | -59.70 | $21,631,134 |
| WOA | Wide Open Agricultur | 0.160 | -3.03 | 6.67 | -50.77 | -25.58 | $28,480,947 |
| AC8 | Auscann Grp Hlgs | 0.040 | 0.00 | 0.00 | 0.00 | 0.00 | $17,621,884 |
| BOD | BOD Science | 0.024 | 0.00 | 0.00 | -72.09 | -82.22 | $4,256,124 |
| BP8 | Bph Global | 0.002 | 0.00 | 0.00 | -50.00 | -88.61 | $2,753,345 |
| CGB | Cann Global | 0.021 | 0.00 | 0.00 | 0.00 | 0.00 | $5,614,845 |
| EVE | EVE Health Group | 0.001 | 0.00 | 0.00 | 0.00 | -50.00 | $5,274,483 |
| EXL | Elixinol Wellness | 0.013 | 0.00 | 0.00 | 8.33 | -40.91 | $8,227,331 |
| IDT | IDT Australia | 0.100 | -9.09 | 0.00 | 58.73 | 31.58 | $36,905,344 |
| LGP | Little Green Pharma | 0.140 | -9.68 | 0.00 | -17.65 | -22.22 | $42,013,053 |
| MDC | Medlab Clinical | 6.600 | 0.00 | 0.00 | 0.00 | -2.94 | $15,071,113 |
| RGI | Roto-Gro Intl | 0.220 | 1900.00 | 0.00 | 0.00 | 0.00 | $4,333,920 |
| ROO | Roots Sustainable | 0.007 | 0.00 | 0.00 | 40.00 | -70.83 | $1,124,217 |
| ZLD | Zelira Therapeutics | 0.920 | -2.13 | 0.00 | -40.65 | -8.00 | $10,439,383 |
| HGV | Hygrovest | 0.050 | -5.66 | -1.96 | 21.95 | -28.57 | $10,515,530 |
| ECS | ECS Botanics Holding | 0.023 | 4.55 | -2.13 | 0.00 | -4.17 | $25,454,805 |
| CAN | Cann Group | 0.096 | -1.03 | -4.00 | -26.15 | -52.79 | $42,432,540 |
| AGH | Althea Group | 0.035 | -5.41 | -10.26 | -16.67 | -43.55 | $14,653,626 |
| MXC | Mgc pharma | 0.490 | -2.00 | -10.91 | -90.20 | -95.55 | $17,880,899 |
| EPN | Epsilon Healthcare | 0.024 | 0.00 | -11.11 | 9.09 | -4.00 | $7,208,496 |
| IRX | Inhalerx | 0.026 | -10.34 | -13.33 | -44.68 | -56.67 | $4,933,941 |
| VIT | Vitura Health | 0.250 | 4.17 | -13.79 | -56.14 | -57.27 | $138,209,709 |
| ME1 | Melodiol Glb Health | 0.002 | 50.00 | -25.00 | -78.57 | -93.18 | $4,728,824 |
| RNO | Rhinomed | 0.030 | 0.00 | -25.00 | -50.00 | -73.91 | $8,571,591 |
| WFL | Wellfully | 0.003 | -25.00 | -25.00 | -40.00 | -81.25 | $1,478,832 |
| WNX | Wellnex Life | 0.025 | -3.85 | -37.57 | -37.57 | -53.40 | $27,074,390 |
Arovella Therapeutic (ASX:ALA)
Arovella announced last week that the Good Manufacturing Practice (GMP)‐grade lentiviral vector for its lead product, ALA‐101, has been successfully manufactured and passed quality release testing.
ALA‐101 was developed to treat CD19+ lymphomas and leukemias.
A key requirement for the development of an iNKT cell therapy product is the establishment of the manufacturing process under GMP conditions.
A critical component for this manufacturing is the GMP‐grade lentiviral vector, which carries the genetic material to program iNKT cells to target and eliminate cancer cells.
The ALA‐101 vector is a 3rd‐generation lentiviral vector manufactured by Lentigen Technology, Inc, a world‐leading manufacturer of lentiviral vectors for cell and gene therapies.
Botanix has successfully completed resubmission of the new drug application (NDA) for Sofpironium Bromide gel, 15% (Sofdra). FDA approval for Sofdra is now targeted for late 2Q 2024.
The resubmission was originally planned for 1Q 2024, but the Botanix team accelerated the process and filed earlier than expected.
The NDA resubmission follows successful completion of the human factors validation study, assessing revised Instructions for Use (IFU) for Sofdra .
Commercial preparations for launch of Sofdra are now underway, as the target for FDA approval has now been moved up to late 2Q 2024.
Emyria said its distinguished psychiatry specialist (unnamed) has been granted “Authorised Prescriber” status by the Therapeutic Goods Administration (TGA).
The authorisation enables the prescribing of MDMA according to an ethics committee endorsed care model developed by Emyria and within the strict regulatory framework established by the TGA for the treatment of Post-Traumatic Stress Disorder (PTSD).
Emyria said that achieving Authorised Prescriber status for MDMA-assisted therapy in PTSD care signifies a strategic step towards expanding Emyria’s service offerings.
Neurotech has completed recruitment for its Phase 2/3 NTIASD2 clinical trial with a total of 56 patients enrolled, all with level 2 (requiring substantial support) or level 3 (requiring very substantial support) autism.
NTI says all patients were enrolled at the Paediatric Neurology Unit at Monash Medical Centre through the trial’s principal investigator Professor Michael Fahey, who is head of the unit and director of neurogenetics.
The trial covers treatment of NTI’s proprietary lead drug formulation NTI164, derived from a unique cannabis strain with low THC and a novel combination of cannabinoids, including CBDA, CBC, CBDP, CBDB and CBN.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








