What’s next for Prana in move from Alzheimer’s to Parkinson’s

Pic: Luis Alvarez / DigitalVision via Getty Images
Leading country and western practitioner Dr Kenneth Rogers says you gotta know when to hold them and know when to fold them.
In the case of Prana Biotechnology, the biotech stalwart has waved the white flag on its Alzheimer’s disease efforts — but it’s far from retreating from the neurology field.
Instead, Prana has turned its clinical focus to two forms of Parkinsonism with no existing treatments and orphan drug designation in the US and Europe.
To do this, Prana is zeroing-in on two proteins in the brain that occur normally, but are over-expressed in sufferers.
Prana’s key weapon is Dr David Stamler, the company’s recently appointed chief medical officer.
At Auspex Pharmaceuticals, Dr Stamler was responsible for the approval of Austedo, or deutetrabenazine, a treatment of chorea associated with Huntington’s disease (chorea is a neurological disorder resulting in jerky movements of the shoulders, hips and face).
>> Brain cancer fighter Patrys is a 10-bagger – which of these noggin-focused stocks will be next?
Auspex was acquired by Teva Pharmaceuticals in mid-2015 for a handy $US3.5billion. Like a pharmaceutical Pied Piper, Dr Stamler took most of his key team with him when he joined Prana in May last year.
“We have someone leading our development with real runs on the board and a strong track record,” says Prana executive chair Geoffrey Kempler.
A brief history of Prana
Mr Kempler started Prana “many years ago” with Bori Liberman, a scion of the billionaire Melbourne Liberman family.
More specifically the company was founded in 1997, based on science developed in-house and with the help of boffins from Victoria’s Mental Health Research Institute, the Florey Institute, the University of Melbourne and Massachusetts General Hospital.
Prana listed on the ASX in 2000 and a Nasdaq listing followed in 2002.
“We wanted to single-handedly cure Alzheimer’s,” Mr Kempler said.
But in early 2014 Prana shares tumbled 70 per cent on news that its imaging trial for Alzheimer’s disease did not meet its primary endpoint of reducing amyloid beta plaques that are implicated in the disease.
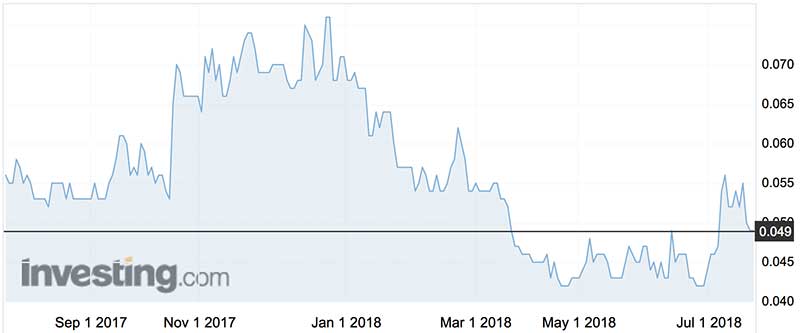
Shortly before that the shares had jumped on news that a phase II trial for Huntington’s disease met the primary endpoint of safety and tolerability (but met only one secondary endpoint, for cognitive efficacy). The net result was that Prana shares returned to their levels of a year previously.
These trials involved a completely different compound called PBT2.
In 2015, Prana said the US Food and Drug Administration had issued a partial clinical hold limiting dosing of PBT2 for patients with Huntington’s disease.
Last year European regulators said they wanted more pre-clinical work before allowing a phase III trial for Huntington’s disease and so that was pretty much the end of that.
Mr Kempler notes that heavyweights such as Pfizer and Eli Lilly have failed to develop Alzheimer’s drugs, with Pfizer walking away from neuroscience altogether.
“It’s been very unfortunate for the patients,” he says. “We continue to be interested in Alzheimer’s disease but in the mean-time we have had to reinvent our strategy.”
The new Prana
The reinvention involves focusing on two forms of atypical parkinsonism: multiple system atrophy (MSA) and progressive supranuclear palsy (PSP).
While Michael J Fox and the late Mohammed Ali made Parkinson’s a household name, PSA and MSA are largely unknown.
Each disorder affects about 10 million people globally including 15,000 to 20,000 Americans, which makes them an orphan disease but at the higher end of the scale.
MSA and PSP sufferers decline more rapidly relative to Parkinson’s disease and both afflictions are eventually fatal. In Dr Stamler’s words, they send the patient into an “unpleasant and inexorable decline”.
MSA patients have difficulty maintain their blood pressure and have bowel and bladder dysfunction, while PSP patients suffer unsteady gait, frequent falls, visual difficulties and cognitive impairment.
PBT434 on trial
In late June, Prana received ethics committee approval for a phase I trial for PBT434 to dose healthy human volunteers.
The trial, which follows the usual mouse and dog stuff, aims to establish safety and test the pharmaco-kinetics. The trial, to be carried out by Nucleus Network Australia, aims to recruit 88 adults aged between 18 and 55 years.
PBT434 is a small molecule that targets alpha-synuclein and tau, intracellular proteins implicated in both Parkinson’s disease and parkinsonism.
Both proteins are good to have in a normal working brain; in fact, without them neurons can’t communicate.
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
But PBT434 was shown to reduce protein accumulation in the brains of rodents induced with the diseases.
This over-abundance is closely related to iron overload.
As Lang Hancock and Gina Rinehart would attest, iron is a nice thing to have but in the case of the body that’s not the case.
PBT434 helps “efflux” iron from the brain cells and returns it to where it should be.
“Like an alpha-synuclein you can’t live without iron,” Dr Stamler says. “It’s just that it has to be in the right place in the right concentration.”
PTB434 is administered orally, but is designed to overcome the pesky blood-brain barrier that has presented so many neurological drugs from working.
Financials and performance
Prana has $17 million of cash, which is unusual because it hasn’t raised any funds for years (but it has received R&D tax incentives).
“We have just been very frugal,” Kempler says, adding the dough should tide the company over for another year.
One Nasdaq American depositary share is worth 60 Australian Prana shares.
Prana shares in the last year have traded between 7.6c (December 2017) and 4.2c (June). They peaked at $1.16 after the Huntington’s trial news in March 2014.
Dr Boreham’s diagnosis:
The company hopes that PBT434 might be developed to the extent that it can tackle Parkinson’s disease as well.
Mr Kempler notes there are many therapies to treat underlying Parkinson’s motor symptoms such as rigidity and tremors. But there’s really nothing approved that will slow the disease.
For the time being, focusing on the orphan indications seems a sagacious strategy because there’s no pending competition and the market is big enough to make money.
“Our preliminary commercial analysis is there is substantial upside if we succeed,” Kempler says.
Orphan diseases also mean a faster path to approval, with Prana expecting to apply for fast-track status … eventually.
The proteins are also being targeted by others, including the Michael J Fox Foundation, Biogen/Roche and Astra Zeneca.
But that’s for the ‘greater’ Parkinson’s rather than the orphan indications.
Meanwhile, PBT2 remains on the books and has potential for partnership or licensing.
Otherwise, PBT434 presents a binary outcome in that it will either work or not work in relation to the two indications.
But given the early stage nature of the clinical work, it will be years before the company has that ‘eureka’ or ‘oh, heck’ moment.
Prana is due to present further evidence of its pre-clinical success at the International Congress of Parkinson’s Disease and Movement Disorders in Hong Kong on October 5.
This column first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner and does not possess a doctorate of any sort. Sadly, he does not own any iron deposits of note.
The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








