What’s next for IDT Australia after moving away from generic drugs

Pic: Charnchai / iStock / Getty Images Plus via Getty Images
Shares in one of Australia’s oldest listed life sciences companies are going for a song, as investors hope IDT Australia can sing a different tune after its recent follies.
IDT’s (ASX:IDT) sub-$20 million market valuation compares with a cash balance of more than $8 million and $19 million of property plant and equipment (a state-of-the-art facility in the Melbourne suburb of Boronia).
The value disconnect has not gone unnoticed, with activist investors Sandon Capital and CVC recently joining the register.
A frank review by board members Graeme Kaufman and Mary Sontrop in February outlined “a cumbersome organisational structure, poor operational execution, manufacturing and operational inefficiencies and under-utilised facilities [at the Boronia plant]”.
But the company is fighting back under a new strategy that has included divesting most of its generics business.
Strategy U-turn
IDT specialises in high potency and difficult-to-manufacture drugs, all made at its Boronia plant.
From initial drug development to clinical trials, IDT is the partner of choice for pharma giants including Pfizer, Johnson & Johnson, Roche, Teva and Bayer.
In 2014, IDT entered the US generics game, paying $US18 million for a package of 23 drugs. At the time, the company touted the deal as a way to increase use of the largely fixed-price Boronia asset and expand margins by making and selling the products.
But a rash of new US generics filings greatly increased competition, so the company was vying with a dozen rivals rather than three or four.
At the same time, the wholesale sector consolidated, which meant IDT was dealing with far fewer buyers.
In April this year, IDT waved the white flag, selling the drug portfolio — except for temozolomide — to ANI Pharmaceuticals for $US2.7 million, plus an ongoing profit-share on one drug.
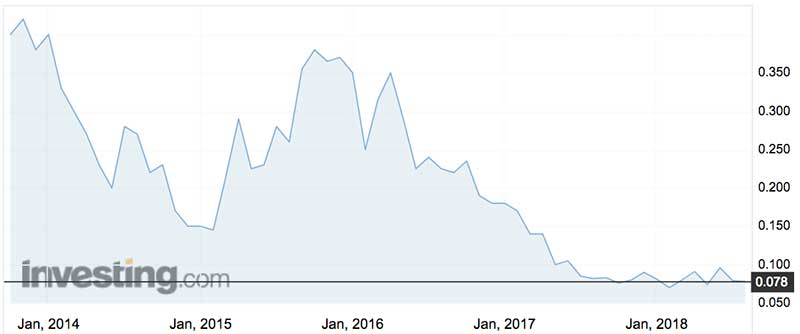
According to the February review, the company saw generics pricing erosion of between 5 per cent and 60 per cent and “the price deterioration continues”. Margins for generics are nowhere near as flash as the original branded product.
Tellingly, of the 23 generics acquired only one was launched and a further three were deemed commercially viable.
IDT’s key remaining generic is temozolomide, an oral brain cancer drug distributed by Mayne Pharma.
Potted history
While your columnist was snug in his mother’s womb in 1965, IDT was conceived thanks to a brainwave from Victorian College of Pharmacy dean Nigel Manning (to create an institute of industrial pharmacy).
As Gough clung to power in October 1975, the Institute of Drug Technology Limited (IDT) was established as an independent company.
While initially an academic-flavored institution, the company developed a reputation for contract drug-making and consulting work.
Under the renowned Dr Graeme Blackman, the company became private in 1982. After acquiring Nicholas Kiwi Central Laboratories, the company listed amid the bicentenary celebrations of 1988.
Dr Blackburn resigned from the board in June last year. Another long-serving director, ex Elders board member Geoff Lord pulled stumps in mid-2016.
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
Thankfully for attendees at the company’s AGMs, the duo’s departures spare the Australian Shareholders Association from having to bang on about the need for board renewal.
With former chief executive officer Dr Paul MacLeman departing in July 2017, the board in February appointed vice prez for business development Dr David Sparling as interim chief executive officer and then anointed him permanently to the role in early July.
The board also appointed two ex big pharma heavy hitters as new veeps: Jim Sosic to oversee operations and Gordon Brien to take care of quality.
Not a love letter
Alas, the Victorian Manufacturing Hall of Fame gong bestowed on IDT in May last year was consigned to the staff pool room after parcel service UPS delivered a plain brown envelope from the FDA in late May, this year.
An FDA warning letter, the epistle outlined quality breaches at the Boronia plant detected during a regular audit in December last year.
The FDA noted “out of specification results and deviations”, a lack of timely corrective actions and “the ability of IDT’s quality systems to ensure integrity of data to support manufacturing of its products”.
On June 6, 2018, the company reported remediation was well underway, including the appointment of a consultant, Seerpharma’s Andrew Giles, who is well-versed in such matters.
IDT also noted there was nothing in the FDA letter precluding the company from exporting its drugs to the US.
“It was a real shock and a blow,” Dr Sparling says. “We will rise to the task and will address the FDA’s concerns. We are working through the process with the FDA now.”
The road to redemption
The February strategic review outlined four options, ranging from a partial generics exit to winding up the company.
As the April sale attests, the board opted to stick with the generics that play on IDT’s experience with cytotoxic drug making (such as the chemotherapy drug thiotepa, a derivative of mustard gas).
Otherwise, IDT plans to beef up its contract manufacturing of both finished drugs and approved pharmaceutical ingredients (APIs).
“The Boronia facility is unique,” Dr Sparling says. “Other entities in the Australian market do some of the things but certainly not all of them.”
Dr Sparling says utilisation at Boronia is improving, to around 30 to 50 per cent, depending on the manufacturing line.
“We have 10 buildings and different manufacturing pods within these buildings. Some will run north of 70 per cent capacity and some will be lower.
“But generally speaking our utilisation and execution is a lot better than what we had been doing over the previous 12 months.”
In a staged process completed in August last year, IDT sold its CMax clinical trials business to a Japanese buyer for a cumulative $16.5 million.
Financially speaking
IDT reported a $17.2 million loss for the half year to December 2017, the result of a $14.1 million impairment of the value of the generics portfolio. But even without this one-off charge, IDT incurred a $3 million operating loss.
But IDT’s balance sheet looks a picture of health with $8.8 million cash, minimum debt and net assets of $31.85 million.
The 12,000 square metres of Boronia freehold land is in the books at a $4.4 million fair value.
This is a conservative number given it was last valued in May 2016 and Melbourne’s industrial values have soared since then. If rezoned to residential the land would fit an untold number of McMansions and an infinite number of apartments.
The company’s property plant and equipment combined is valued at $18.91 million (as per the half-year accounts) but the replacement value is more like $50 million to $70 million.
IDT is carrying $25.8 million of tax losses – a nice asset for a buyer that could engineer a way to use them.
Dr Boreham’s diagnosis:
IDT shares peaked at just under $2 in September 2008 and are just off their February 2018 record lows of around 7c.
But as Frank Sinatra said, “That’s Life” and things look to be on the up. The company says it is meeting the industry standard of 90 per cent-plus schedule adherence and on-time delivery.
In the June 6 update on the FDA’s concerns, IDT also reported a strong order book up to June 30.
As for Sandon and CVC’s brooding presence on the register, we understand neither fundie has made any particular requests (or demands) of management.
IDT is not the only Aussie biotech to come a cropper in the US generics market.
Just ask generic king Mayne Pharma, which reported an impairment laden $174 million first-half loss. Or generics aspirant Acrux, which is trading at close to its cash backing.
IDT’s full year results, to be released next month, should show at least some evidence of a turnaround.
This column first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner and does not possess a doctorate of any sort – generic or otherwise.
The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








