Invitrocue is promising cheap, personlised cancer tests

Pic: Charnchai / iStock / Getty Images Plus via Getty Images
As a provider of bespoke drug tests Invitrocue is an exponent of personalised medicine — which is not snuggling up with a bottle of penicillin, but tailoring therapeutics to suit the individual.
In the case of oncology, Invitrocue (ASX:IVQ) is not claiming be on the cusp of curing cancer.
But its 3D-based diagnostic technology is helping clinicians work out the best drugs to administer more quickly.
Singapore-based Invitrocue’s flagship product is Onco-PDO, as in oncology patient-derived organoid.
Onco-PDO uses 3D culture to grow a patient’s own cancer cells on scaffolds and then test them in view of the best medicine to use.
The technology is based on the notion that every tumour is slightly different, which means individual patients may react differently to a particular drug or drug combination.
“We create micro-tumours that are highly correlated to the patient’s cancer tumour,” company founder and chief executive officer Dr Steven Fang says.
In effect the process creates a 3D tumour “avatar”, so let’s think of Invitrocue as the Lara Croft or Dr Grace Augustine (Sigourney Weaver) of the oncology world.
Invitrocue already has two products for in-vitro liver toxicology testing: Hepatocue and 3D Cellusponge.
While the oncology product is used by clinicians, the liver testing is deployed by pharmaceutical companies to assess the toxicity of new drugs.
The market is a buoyant one given the ageing population and lack of effective cures for non-alcoholic steatohepatitis (NASH) and hepatitis B.
In 2016, Invitrocue expanded its work from liver diseases to oncology.
Onco-PDO is competing with the gold-standard technique of the xenograph mice model (patient-derived tumours grown on the animals).
The trouble is, each of these specially-bred, immune system-deprived rodents costs $15,000 to $20,000. So one just can’t visit a Mallee wheat silo with a toss bag to grab a few.
Invitrocue claims the advantage of being significantly more cost-effective, with a much faster turnaround of days rather than weeks.
In mid-April the company said it had screened its first patient with Onco-PDO for colorectal cancer, generating first revenue for the product.
That’s worth a drum roll.
Dr Fang says the company is getting daily inquiries from customers in relation to patients who are failing their therapies and newly-diagnosed diseases.
‘We are not a biotech’
Dr Fang dubs Invitrocue as a life science or healthcare company, rather than a biotech.
That’s because these days it spends little on research and development. But the company has benefited from $40 million sunk into the technology in the past.
“The capital required now is about building capacity to roll out the services,” he says.
Dr Fang founded Invitrocue in 2012, based on technology that originated within Singapore’s august Agency for Science Technology and Research (Astar).
This discovery was led by Prof Hanry Yu, at Astar’s Institute of Bio-engineering and Nanotechnology.
The company back-door listed in June 2015, having been acquired by the shell of Bunuru Corp.
(As an aside, in its previous life Bunuru was linked with a scandal involving the Panama Papers and mining companies operating in North Korea).
A former Glaxosmithkline heavy-hitter, Dr Fang has listed no fewer than four companies over the years.
The last one, the umbilical cord stem cell blood bank Cordlife remains listed on the Singapore Stock Exchange with a $190 million market cap.
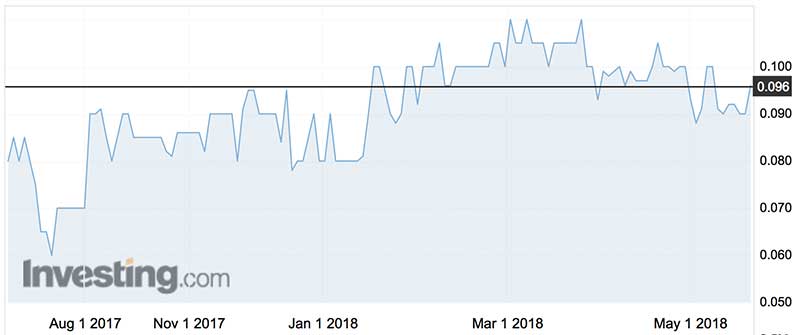
Commercialisation plans
Invitrocue so far has focused on its Asian home base, as well as Japan, Hong Kong, Australia and Europe.
“We haven’t even started looking at the US,” Dr Fang says. “We are doing every country we can physically get our head around, rather than doing everything and not succeeding.”
Invitrocue’s revenues to date have derived from the liver testing. These amounts include reagents and consumables, as well as revenue for interpreting the data the models generate.
The company expects to roll out its Onco-PDO kits this year.
In February, the company announced $100,000 assistance from Invest Northern Ireland, which could involve “a number of possible scientific and commercial partnerships”.
It’s expected this tie-up will be a beach head to the European market — even though Irish eyes aren’t smiling after Northern Ireland is being forced to secede from the EU by its Westminster masters.
Invitrocue isn’t ignoring the Middle Kingdom, either: In August 2016, the company inked a strategic agreement with Qiagen Suzhou Translational Medicine Co.
This deal involves Invitrocue providing its technology to discover biomarkers (indicators of disease) for the Chinese biopharmaceutical market.
Financials
Invitrocue’s is light-on for cash — with just $1.7 million at the end of March — but the directors opine the company is a going concern as it intends to raise “at least” $2.5 million by May 2018.
Hey – that’s this month!
Dr Fang confirms a capital raising is in train, but is circumspect about its exact nature.
In the December quarter, Invitrocue raised $2.6 million from institutional investors across two placements.
The company also has 69.7 million unlisted options on issue, exercisable between 5c and 10c, with 48.4 million expiring in February 2033, about the same time as Dr Augustine’s ship leaves for Pandora in search of unobtanium.
The board is also mulling a dual listing but not necessarily on the Nasdaq, having been contacted by a number of exchanges globally.
“But my hope is to make Invitrocue a dividend stock before we talk about corporate action,” Dr Fang says.
Dividend? Say that again?
Yes!
Dr Fang says that Invitrocue can become profitable even without Onco-PDO and pay a divvy — a unicorn-type rarity in the biotech, sorry, life sciences, world.
“But Onco-PDO will really drive the company in terms of revenue and profits.”
Dr Boreham’s diagnosis
Invitrocue is a bit like the 19th Century goldfields shovel merchants, who ultimately did better than the miners themselves.
Rather than digging for gold (making drugs) per se, Invitrocue is making life easier for the drug-makers while hopefully saving lives.
As usual, there are grown-up companies that Invitrocue can aspire to become. The Nasdaq-listed Foundation Medicine tests for more than 300 cancer-related genes and is worth a cool $US2.5 billion.
Dr Fang says he’s made mistakes along the way and Invitrocue will learn from them.
“There is no such thing as getting it right in the first time.”
That said, Invitrocue looks well-past the experimental sand-pit stage and hopefully that will be reflected in the financials sooner rather than later.
This column first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner and does not possess a doctorate of any sort. Mistakes? He’s made a few — often the same ones twice — and is still learning from them, but looks good in blue skin.
The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.