Tim Boreham (and Acadia) go through Neuren’s undies drawer

Pic: Luis Alvarez / DigitalVision via Getty Images
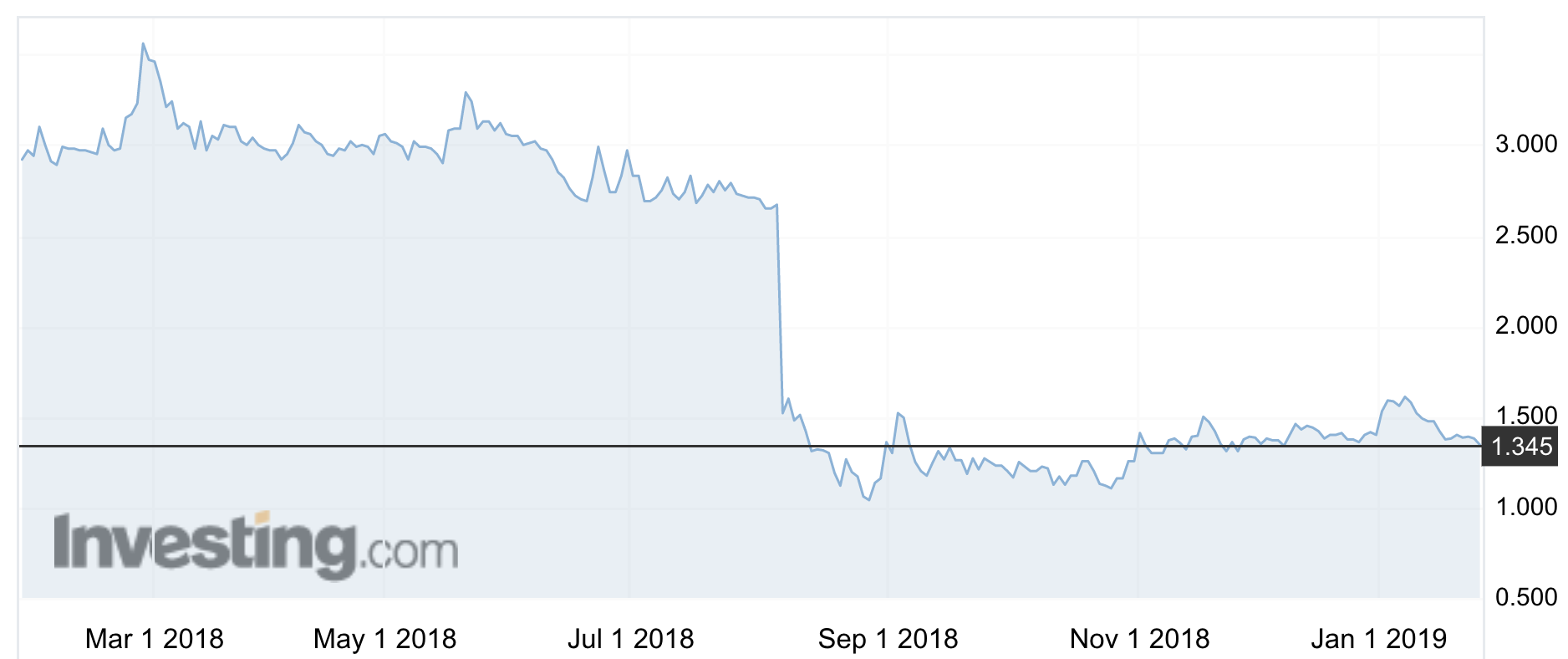
Six months after Neuren struck a company-changing partnering deal to develop its key asset Trofinetide — formerly known less romantically as NNZ-2566 — shares in the neurodegenerative disorders house are trading at about half their pre-deal value.
While most CEOs pretend not to follow their companies’ daily share price fluctuations, Neuren’s Dr Richard Treagus is openly frustrated about the market’s reaction to the deal with the San Diego-based Acadia Pharmaceuticals, which has a headline value of $US465 million ($660 million).
“Fundamentally we are in a much better position now,” Dr Treagus muses.
“It makes you contemplate whether we are doing the thing that matters in terms of getting a drug progressed to market.”
We presume the answer is ‘yes’, despite the lukewarm response from Mr Market.
The deal gave Acadia the North American rights to Trofinetide, which is targeting the rare neurological conditions Rett syndrome and Fragile X syndrome. Crucially, Acadia funds the estimated $US55 million cost of a planned 180-patient phase III trial for Rett syndrome, to start in the second half of 2019.
A form of autism, Rett syndrome affects about one in 10,000 females and is characterised by intellectual disability, loss of motor control and muscle rigidity. Fragile X is an inherited chromosome mutation known as a cause of autism, affecting about one in 4,000 males and one in 6,000 females.
Despite Dr Treagus’s frustrations, Neuren (ASX:NEU) isn’t about to pack it in and join the pot stock or Bitcoin brigade.
Quite the contrary in fact: now that Acadia has assumed the cost of the phase III trial of Trofinetide, the company can hop to work on its second drug candidate, NNZ-2591 for autism spectrum disorder.
Rest of the world deal pending?
To recap, the $US465 million headline Acadia deal delivered Neuren an upfront fee of $US10 million, with a further $US105 million in development milestones and up to $US350 million of sales-based payments. Neuren also receives double-digit royalties.
There’s also another potential source of revenue for Neuren in the guise of a Rare Paediatric Disease Review Voucher, an FDA concession aimed at providing drug makers with an incentive to develop new drugs.
Drug makers with an approved product may quality for the Willy Wonka style vouchers, which can be redeemed to receive a priority review of a different product. They can also be sold or transferred, with the vouchers changing hands for between $US110 million and $US150 million.
Under the deal with Acadia, Neuren gets one-third of any spoils.
While the afflicted population is small, Acadia expects US sales of $US500 million a year plus.
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
Then there’s the rest of the world, to which Neuren has retained the rights.
But not for long, perhaps: in early November, Neuren and Acadia entered three-month exclusive negotiations for these rights, with the exclusivity period expiring on January 31.
Yes – this week!
“They’ve really been going through the socks and undies draw,” Dr Treagus says of Acadia’s evidently thorough due diligence of the company’s ‘smalls’.
Acadia received unwanted publicity with safety concerns over its Parkinson’s drug Nuplazid (pimavanserin), but in September the US Food and Drug Administration delivered the anti-psychotic drug a clean bill of health (being a good mate, Neuren helpfully disclosed this in its own ASX announcement).
In October, Acadia announced positive phase II results for the drug, which Neuren says “quite clearly demonstrates Acadia’s capabilities in neurology drug development”.
If a global forthcoming is not forthcoming, Neuren will still enjoy the advantage of full and free access to any of Acadia’s clinical material pertaining to Trofinetide.
Trofinetide, by the way, helps to restore equilibrium in the brain and reduce inflammation. It is a synthetic version of the neurotrophic peptide insulin-like growth factor-1 (IGF-1), a growth factor produced by brain cells.
Lest we forget NNZ-2591
Dr Treagus says NNZ-2591 has been a “footnote” in the company’s activity statement, because the company didn’t have the “bandwidth or money” to further it.
“It’s a white space,” Dr Treagus says of autism. “There are no drugs.”
“We view autism spectrum disorder as a group of diseases with some element of autism. We are looking to opening an investigational new drug application with the FDA for human trials.”
Finances and performance
Apart from the general stock malaise, Neuren’s share slump post the Acadia deal stems from the market’s perception the deal is overly back-ended (others are criticised for giving too much away for too little, too early, so it’s hard to strike the balance).
“The only disappointing part of the deal was the market’s reception,” Dr Treagus insists.
In November 2017, the company underwent a 20-for-one share consolidation, with the shares trading between $1 and $3.60.
Some investors appear to be discounting the benefit of Acadia assuming the cost of the phase III trials.
In May, Acadia showed the color of its money by spending $US4 million on 1.3 million Neuren shares at $4 apiece, a 30 percent premium to the prevailing share price.
Acadia last November raised $US316 million, so there’s no doubt it has enough folding stuff to fund the trofinetide trial.
Post the deal, Neuren has cash of $24 million (as of the September quarter) with very few spending commitments. “We certainly don’t need to raise any money,” Dr Treagus says.
On that note, the then cash-strapped Neuren entered a funding deal with global firm Lanstead Capital in July 2017. In effect a staged share placement, the deal involved $1.5 million with a further nominal $8.5 million delivered over 18 months with the value dependent on Neuren’s share price.
A base price of 8.86c a share was struck — equivalent to $1.77 post consolidation — which means that at current valuation of $1.38 Neuren receives less rather than more.
The final payment to Neuren was due in February this year, but given the lowly share price, last August the company deferred this payment until June.
Dr Boreham’s diagnosis:
Share valuation aside, Neuren has indeed come along since Dr Boreham applied a full body examination to the stock in March 2017.
At the time, Neuren was recovering from the failure of its Intrepid clinical program to treat traumatic brain injury.
Neuren’s auditor also kindly pointed out a “material uncertainty” if it couldn’t get hold of more cash.
Financially, Neuren has the advantage of being backed by property developer Lang Walker, who has invested in other ASX biotechs including the once promising but ultimately failed drug developer QRX Pharma.
Neuren is ably led by Dr Treagus who ran the transdermal oestrogen and testosterone treatment house Acrux in the company’s more virile days.
In the short term, the outcome of the global rights negotiations for trofinetide is a key driver, while investors can also expect management to outline the NNZ-2591 program.
The company’s December 4C (quarterly) update lobs next week, while investors should also expect news on the rest of the world Trofinetide rights.
“I think 2019 will be a good year for us,” Dr Treagus says.
This column first appeared in Biotech Daily
Disclosure: Dr Boreham is not a qualified medical practitioner and does not possess a doctorate of any sort. Rest assured, his undies drawer is spick and span and should pass Ms Crucible’s due diligence.
The content of this article was not selected, modified or otherwise controlled by Stockhead. Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








