This ASX stock is watching you while you sleep… because it could point to Parkinson’s disease

Acting out your dream could be a sign of Parkinsons. Picture Getty
- Acting out your dreams could be a sign of a neurological disease, a study finds
- ASX-listed Pharmaxis to launch a study on iRBD patients who are at risk of Parkinson’s
- Stockhead reaches out to Pharmaxis CEO, Gary Phillips
A rare sleep disorder that makes people act out their dreams may be an early warning sign of a neurological disease.
According to the study, over 70% of people who suffer from isolated Rapid Eye Movement (REM) Sleep Behaviour Disorder, or iRBD, transitioned to a neurodegenerative disease like Parkinson’s or Dementia with Lewy Bodies.
iRBD is a condition characterised by muscle atonia (a loss of muscle tone) that occurs during the REM stage of sleep.
It causes otherwise healthy people to act out their dreams, which can often result in serious injury to themselves and their bed partner.
So what’s the link between iRBD and Parkinson’s?
Although much is still unknown, we do know that Parkinson’s starts out by affecting the lower part of the brain stem before it progresses very slowly up to the basal ganglia – the part that controls coordination of movements.
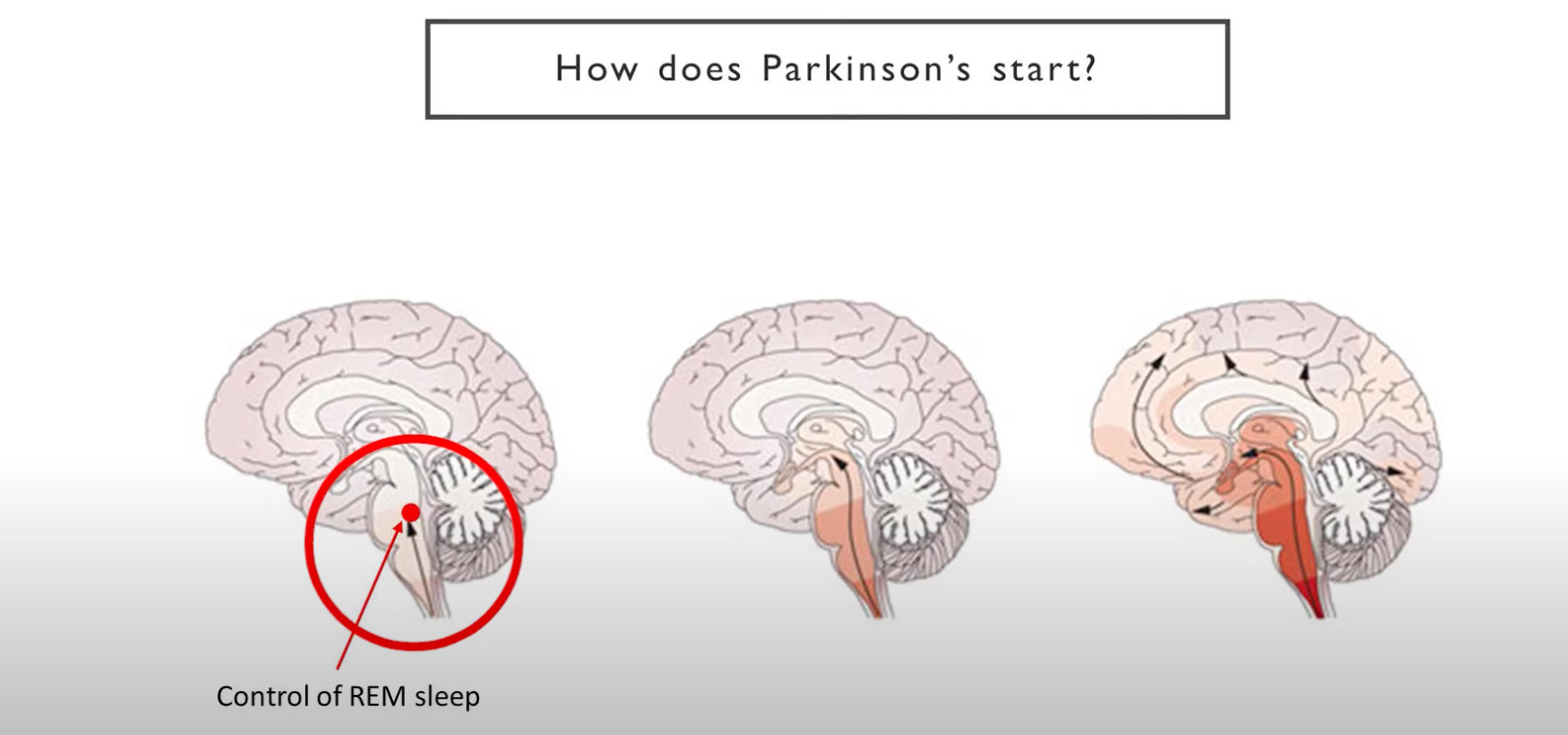
This is the stage where you start seeing the motor symptoms of Parkinson’s – the tremor, stiffness, and rigidity. From there, the disease often progresses further up where it affects things like memory.
But as you can see, it has to travel quite a long way up before you start getting those symptoms.
At a much earlier stage however, the disease affects part of the brain that controls REM sleep (red circle).
Several studies have thus concluded that a REM sleep behaviour disorder can be one of the earliest symptoms of Parkinson’s, sometimes occuring years before any motor symptoms.
Pharmaxis to launch Phase 2 trial
Aussie biotech Pharmaxis (ASX:PXS) is about to launch a study into iRBD patients who are at risk of Parkinson’s and other neurodegenerative diseases.
The company has just received a £2.9m (around $5m) funding from Parkinson’s UK to conduct a Phase 2 trial investigating the effect of its proprietary drug PXS-4728 on iRBD patients.
This ‘groundbreaking’ study will see Pharmaxis collaborate with leading neurologists from Sydney and Oxford universities.
Pharmaxis CEO, Gary Phillips, explained to Stockhead the importance of diagnosing Parkinson’s early.
“By the time you get diagnosed with Parkinson’s, you’ve lost something like 80% of the neurons, which are the source of the dopamine, the bugs that cause the Parkinson’s symptoms,” Phillips said.
“So the horse is already gone and you’re trying to shut the stable door, so to speak.”
Phillips said the holy grail is to identify these patients early on, before they start this line of cell death in the brain.
“The belief is that if you can treat the inflammation in the brain, then you can stop the cell death, and in turn slow or even stop the progression to Parkinson’s,” Phillips said.
Accidental find
The most intriguing part of the study is that PXS-4728 wasn’t even developed to treat neurological diseases in the first place.
The drug was initially developed by Pharmaxis as a potent inhibitor of the inflammatory enzyme SSAO (semicarbazide‐sensitive amine oxidase).
It was licensed in 2015 to Boehringer Ingelheim (BI) for $83m upfront, and was extensively studied in 11 clinical trials ranging from liver diseases to diabetic retinopathy.
“BI put huge amount of investment into developing the product,” Phillips told Stockhead.
But despite promising results, Boehringer returned the drug to Pharmaxis in late 2020 due to an unexpected effect on an additional inflammatory enzyme in the brain, MAO‐B (monoamine oxidase B).
“That gave them a problem because it became a risk factor to developing the drug in liver disease,” explained Phillips.
Phillips estimated that in total, BI had invested well over US$100m into PXS-4728 – which was a lot of value for Pharmaxis shareholders to benefit from if it could only be repurposed.
He began to search for ideas, and a few meetings with local neurologists in Sydney quickly narrowed the search to neurodegenerative diseases where neuro inflammation is thought to play a key role.
“It was an obvious step to focus our attention and repurpose PXS-4728 on neurology indications,” said Phillips.
“It’s a bit parallel to the story of selindifil, which was developed for pulmonary hypertension but had one unforeseen side effect that turned the clinical development program around and gave birth to Viagra.”
The PXS-4728 study will examine whether targeting inflammation in the brain of people with iRBD might provide a viable neuroprotective strategy to prevent the disease.
Experts from the University of Sydney and the University of Oxford will recruit 40 patients with iRBD to participate in the placebo‐controlled Phase 2 trial.
According to Phillips, targeting patients with iRBD offers the best strategy for slowing cell death, as we have no other disease modifying treatments for Parkinson’s disease at the moment where most diagnoses are usually too late.
“With Parkinson’s having the unwelcome tag as the fastest growing neurological condition, plus the interest in the program I have seen from biotech and pharma companies that have a CNS (central nervous system) portfolio, I believe there is a significant commercial opportunity here,” said Phillips.
Pharmaxis share price today:
ASX stocks with Parkinson’s/neurological disease programs
Pharmaxis (ASX:PXS)
Neurodegenerative diseases are actually not an area of focus for Pharmaxis, and the company is currently running four concurrent clinical trials in other indications.
The Phase 2 clinical trial of PXS-5505 on myelofibrosis is its lead program, with more than 50% of patients recruited right now.
PXS-5505 will also commence a US investigator-led Phase 2 trial in liver cancer in Q3 this year.
Meanwhile, a Phase 1 trial of PXS-6302 on burn scar treatment is being conducted with Dr Fiona Woods – the surgeon named Australian of the Year in 2005 for her work with the Bali bombing patients.
On top of that, another trial in PXS-6302 in scar prevention will commence recruitment in the first half of next year of 2023.
Phillips says it’s an exciting and busy time for Pharmaxis with a lot of newsflow to come before year end.
“Given where our valuations are at the moment, this is probably a good time to look at us and assess whether you want to get in ahead of the results, or you want to wait and see what the results are,” said Phillips.
Alterity Therapeutics (ASX:ATH)
While available therapies address the symptoms of these neurodegenerative disorders, Alterity is targeting the underlying pathology of the disease.
The company’s lead drug candidate, ATH434, is designed to inhibit the aggregation of alpha-synuclein, a protein implicated in neurodegeneration.
The drug was also designed to preserve nerve cells, and improve function by restoring normal iron balance in the brain.
Alterity has just announced the UK launch of its Phase 2 clinical trial of ATH434 in Multiple System Atrophy (MSA), a rare Parkinsonian disorder.
Living Cell Technologies (ASX:LCT)
NTCELL is an investigative disease-modifying therapy for Parkinson’s disease under development by Living Cell Technologies (LCT).
The unique cell therapy consists of a capsule that contains newborn choroid plexus cells from pigs without pathogens bred from a stock discovered in the sub-Antarctic Auckland Islands.
Phase 1 results demonstrated safety and efficacy, while Phase 2 has met its primary efficacy clinical endpoint.
LCT has now entered a new phase in its research, with the announcement in May that it would be applying the benefits of artificial intelligence (AI) to ensure the NTCELL product being manufactured is of the highest possible quality.
To facilitate this research, the company signed an agreement with Sydney-based start-up OptiCellAI, which is led by award-winning engineer Michael Urch (CEO) and clinical embryologist Tamara Treleaven.
The first trial participants are expected to receive treatment in 2024.
Emyria (ASX:EMD)
Emyria has made strides in its MDMA analogue program and has initiated pre-clinical studies.
In 2021, the company partnered with the Western Australian University (UWA) to initiate a program that aims to establish a large drug candidate library.
The library now comprises over 120 novel MDMA-like compounds, with three drug discovery priority areas identified.
These priority areas include mental health disorders and neurological disorders such as Parkinson’s disease.
Emyria is also one of three health and biotech companies to receive a significant multi-million dollar cash injection from Andrew Forrest’s private investment vehicle Tattarang.
Neuroscientific Biopharma (ASX:NSB)
NSB has successfully transitioned into clinical trials for its lead drug, peptide based compound, EmtinB, which was designed to treat neurodegenerative conditions like Multiple Sclerosis and Alzheimer’s disease.
EmtinB has the potential to address this largely unmet medical need by using its novel mode of action to harness the body’s own defence mechanisms and combat the progression of the disease.
An early-phase clinical trial is now underway, which aims to develop biomarker data from human blood samples.
NSB is also undertaking animal studies for multiple sclerosis, and has already reported very promising preliminary results from one study.
Actinogen Medical (ASX:ACW)
Actinogen has fully funded Phase 2 trials in its Xanamem pipeline targeting three CNS (central nervous system) indications, with readouts expected in 2022/2023.
It includes the Fragile X Syndrome trial, which is being expanded to sites in North America to accelerate enrolment timeline.
Actinogen is also pursuing trials in depression, having initiated a Phase 2 study in Australia.
In addition, it’s also looking into Alzheimer’s disease by studying the effectiveness of Xanamem in targeting and blocking the production of cortisol inside neurons (cells) in the brain.
Neuren Pharma (ASX:NEU)
Neuren is developing new therapies for debilitating neurodevelopmental disorders that emerge in early childhood.
The company owns two novel drugs, treating six neurodevelopmental disorders, all with Orphan Drug designation.
It owns all of the intellectual property to these drugs, with no royalties payable to third parties.
In December last year, Neuren announced that it was close to launching the first ever drug for Rett syndrome to the market.
Following that study, its US partner Acadia has submitted a New Drug Application (NDA) to the US FDA, and is currently waiting for an approval.
Share prices today:
At Stockhead we tell it like it is. While Neuroscientific Biopharma is a Stockhead advertiser, it did not sponsor this article.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








