The Ethical Investor: Why Starpharma and these ASX biotechs could stop the next infectious disease outbreak

Why Starpharma’s Viralzee could stop the next outbreak. Picture Getty
- The infectious diseases space is one area that could be interesting for ethical investors
- An ASX company that already has a product in the market is Starpharma
- Stockhead reached out to Starpharma CEO, Jackie Fairley
Throughout history, humanity has struggled with infectious diseases and the subsequent epidemics and pandemics.
While the discoveries of antibiotics and vaccines have significantly reduced the number of deaths over the last 100 years, several factors have recently led to a resurgence of known infectious diseases.
These factors include climate change, urbanisation, social inequality, migration, and wildlife consumption – to name a few.
The World Health Organization (WHO) estimates that globally, infectious and parasitic diseases caused 5.1 million deaths in 2019, of which nearly 52% (2.64 million deaths) were due to eight major diseases below:
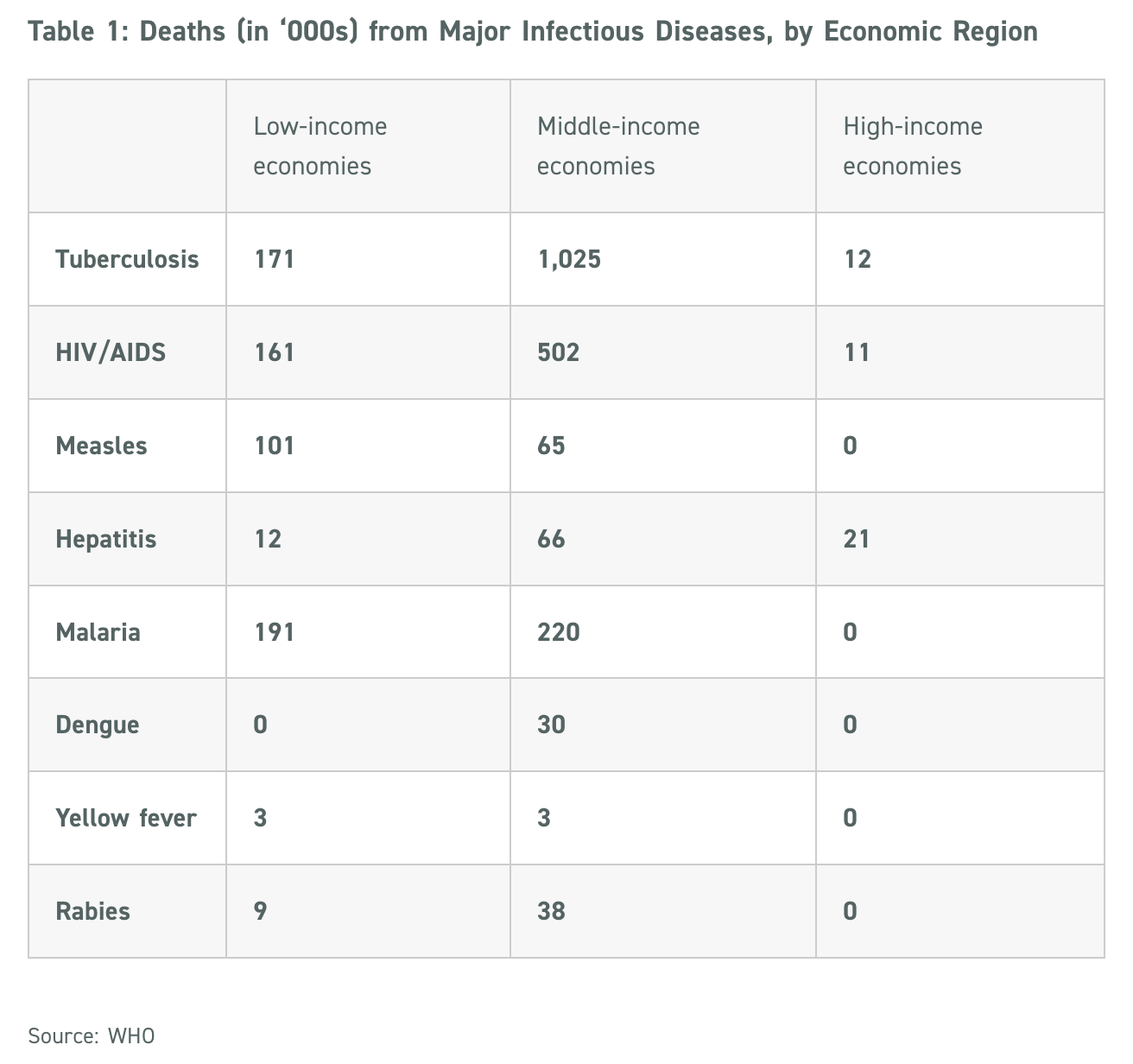
These eight infectious diseases, classified as NTDs (neglected tropical diseases), cause not only deaths, but also debilitating losses to trade, travel, livestock – and thus the overall economy.
And when they’re combined with other infectious diseases such as MERS, Ebola, Zika, Nipah, and COVID-19, the economic burden is severely amplified.
For investors looking into biotechs, experts believe there is a clear opportunity and a sizeable market in the infectious diseases space.
According to a report by ISS Insights, only 13% of biotech companies worldwide are currently involved in R&D for novel therapies to address the challenge.
“The substantial threat in economic terms posed by infectious diseases presents a huge opportunity to drive global health and prosperity,” said the report from ISS.
“An investor looking to drive significant social impact through infectious disease control may wish to consider pharmaceutical companies performing R&D in infectious diseases affecting low-and middle-income countries.”
Starpharma may be one to watch
An ASX company that already has a product in the market to combat infectious diseases is Starpharma (ASX:SPL).
The company’s flagship is Viraleze, a nasal spray that contains an antimicrobial/anti-infective polylysine dendrimer called SPL7013, which was developed in-house.
Once applied, Viraleze forms a protective mucoadhesive barrier in the nose where most cold and respiratory viruses first take hold.
SPL7013 in Viraleze then fortifies the barrier between the viral particles and the nasal cell membrane, effectively trapping viruses and blocking them from infecting the human cells.
SPL7013 has been shown in laboratory studies to be effective against a broad spectrum of respiratory viruses, including Delta and Omicron, as well as influenza viruses, rhinoviruses, SARS and MERS viruses, and respiratory syncytial virus (RSV).
The active ingredient’s mechanism is rapid, achieving more than 99.9% reduction of viral load within a minute of contact.
Starpharma CEO, Dr Jackie Fairley, explains that an intervention such as Viraleze is important for stopping a virus at the beginning of infection, in the nose, so the virus can’t take hold or migrate to other parts of the body, such as the lungs, and cause people to get sick.
“Viraleze sprayed in the nose thus has the potential to reduce infections and community transmission of a wide range of viruses, including those responsible for recent pandemics,” Fairley told Stockhead.
Ready for the next pandemic
COVID-19 is not the first pandemic the world has faced, and it won’t be the last. Indeed, you could say that we are now “between pandemics”.
According to Fairley, antiviral measures such as Viraleze will remain important for as long as people continue to contract viral respiratory infections, whether it be a cold, seasonal flu, or a future pandemic virus.

“A key advantage of Viraleze nasal spray is its broad-spectrum antiviral activity, which makes it a promising tool for pandemic preparedness strategies that account for the emergence of as-yet-unknown viruses,” she said.
In addition to being an everyday product for colds or flu, Viraleze also represents an important tool for patients who are immuno-compromised and don’t effectively respond to vaccines.
“We know that such patients and medical professionals seek access to preventative measures beyond those that are currently available.”
Starpharma has registrations for Viraleze covering more than 35 countries, including Europe, the UK, and several Asian countries. The company has recently secured a regulatory approval in Malaysia.
“Starpharma’s strategy for Viraleze is to focus on commercially attractive consumer markets and countries with rapid regulatory pathways,” said Fairley.
The company has already attracted partnerships with three of the world’s largest pharmaceutical companies – AstraZeneca, MSD and Genentech.
In addition to Viraeleze, Starpharma also has several oncology drugs under development and multiple other products in the market.
“We’re well-funded to advance our product development pipeline, with a number of key milestones on the horizon,” said Fairley.
“These include upcoming development milestones with partners, results from multiple oncology trials, and expanded commercial markets for Viraleze.”
Starpharma share price today:
Other ASX stocks in the infectious diseases space
Island Pharmaceuticals (ASX:ILA)
Island develops antiviral therapeutics to address infectious diseases.
The company’s lead asset is ISLA-101, a drug with a well established safety profile, being repurposed for the prevention and treatment of dengue fever and other mosquito (or vector) borne diseases.
The company is moving rapidly into clinical trials for dengue-infected patients.
Last month, Island announced that the US FDA has cleared ISLA-101’s Investigational New Drug application, enabling clinical progress to proceed.
Clinical dermatology company Botanix was granted Qualified Infectious Disease Product (QIDP) by the US FDA Office of Antimicrobial Products.
The new QIDP status covers the use of of BTX 1801 for the “reduction of risk of S. aureus bloodstream infections in colonised patients on central venous catheter-dependent hemodialysis.”
BTX 1801 is a synthetic cannabidiol (CBD) gel, delivered to the patient using a permetrex skin delivery technology.
Immuron focuses on the development of novel polyclonal antibodies to treat diseases associated with the gastrointestinal tract.
The company’s flagship product is Travelan, an orally administered immunotherapy that reduces the likelihood of contracting travelers’ diarrhoea.
It’s a listed medicine on the Australian Register for Therapeutic Goods, where it is indicated to reduce the risk of travelers’ diarrhoea and minor gastro-intestinal disorders.
Recently, the company received approval from the US Army to proceed with the clinical trial to evaluate the efficacy of Travelan to prevent infectious diarrhoea caused by enterotoxigenic Escherichia coli (ETEC).
Invion is developing a technology called Photosoft, used for the treatment of a range of cancers, atherosclerosis and infectious diseases.
Photosoft is billed as a novel next generation Photodynamic Therapy (PDT) that uses non-toxic photosensitisers.
In the first half of FY23, Invion achieved multiple promising results from early studies using Photosoft on several infectious diseases, most notably on the antibiotic resistant MRSA bacteria.
GSS is a molecular diagnostics company focused on the detection of infectious diseases using its proprietary platform technology, 3base.
3base is a molecular test that detects the DNA or the RNA of an organism. GSS designs and manufactures the test kits based on this technology.
Previous studies have concluded that the kit’s “short turnaround time and simplicity makes it suitable for routine use in most clinical microbiology laboratories”.
The views, information, or opinions expressed in the interview in this article are solely those of the interviewee and do not represent the views of Stockhead.
Stockhead has not provided, endorsed or otherwise assumed responsibility for any financial product advice contained in this article.
Related Topics
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








