The ASX had questions for Biotron about that big HIV announcement

Stockmarket authorities have raked over small cap biotech dazzler Biotron’s ASX announcements in the wake of its rollercoaster run over the past month.
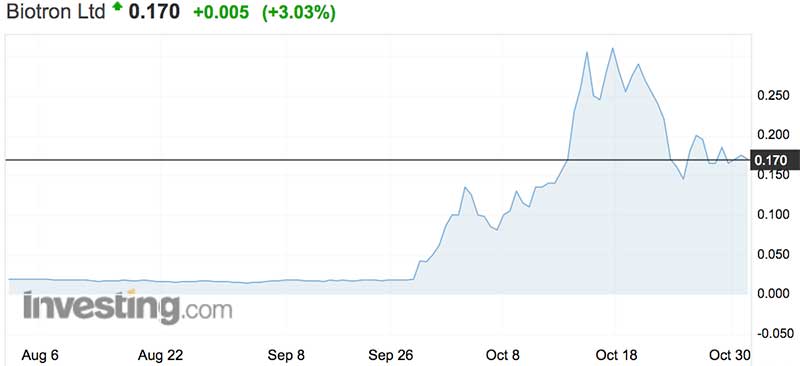
Biotron (ASX:BIT) hit 20-bagger status this month after announcing its flagship drug BIT225, had “significant immunological outcomes” in a Phase 2 clinical trial.
The test results were “a major step to the ultimate goal of curing HIV-1 infection”, Biotron boss Dr Michelle Miller said at the time.
Prior to the news, BIT shares were trading at 1.9c a pop. At their peak on October 17, they reached 44.5c before cooling to a more sedate 17c on Tuesday.
Last week the ASX sent a six-page query to Biotron with 15 questions about the anouncements.
The ASX sought information on benchmarks used in the trials, measurement techniques, the identity of scientists who took part in the trial and compliance with Listing Rule 3.1 (which requires a listed entity to publish immediately any information that could have a material effect on the share price).
The ASX pointed to its Code of Best Practice for Reporting by Life Sciences Companies, which requires companies to report “regardless of whether the outcome is positive or negative, should be clear and unambiguous, specifically addressing the endpoints announced at the commencement of the trial”.
Biotron responded today with its own six-page response, confirming it was in compliance with Listing Rule 3.1.
Investors backed the company in early trade, sending it up 21 per cent to an intraday high of 20c, before dropping back to 16.5c shortly before market close.
Biotron said it had disclosed all information on September 28 previously via the proper channels but was unable to release some information that remained trade secrets.
Full results of the trial would be released publicly following presentation at an undisclosed “upcoming scientific conference in the USA in late 2018 / 2019”.
The company has been contacted for comment.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








