Take 2: BARD1 gets its research right, investors like what they see

Pic: Getty
BARD1 Life Sciences has re-proved its cancer diagnostics technology, after a rushed study in 2017 delivered some disappointing results.
The study tested its ovarian cancer detection technology and algorithm on 400 blood samples, and the company said they had high rates of accuracy.
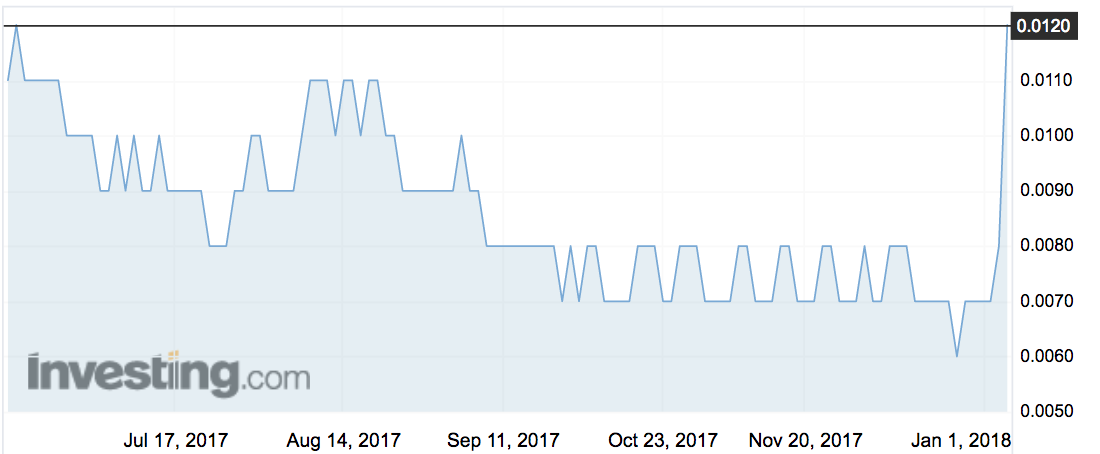
The company’s stock (ASX:BD1) closed up 50 per cent on the news, to 1.2c.
BARD1’s technology uses a biomarker to detect the body’s autoantibodies which attack abnormal proteins produced by cancerous tumours. The results are fed through an algorithm which provides a “cancer score”.
The score had an 82 per cent sensitivity – that is, the number of patients correctly identified as having cancer – and 79 per cent specificity, the number of people correctly identified as not having cancer.
In early last year, the same technology was used in a lung cancer study by Meso Scale Diagnostics but the results were disappointing.
CEO Leearne Hinch criticised the method, which rushed into the study before optimising the method for the tech, and had a possible gender influence.
The results meant they had to re-prove the concept.
They brought on a new science advisor and a consultant to rejig the study design and fix the problems around patient selection, the number of cancer samples versus those from healthy people, and the method itself.
“For the company now the focus is on moving forward with both [ovarian and lung cancer] of these tests,” Ms Hinch told Stockhead.
“But because it’s the same technology we will take one test forward, build an assay on one commercial platform… and then we’ll change the biomarkers to build an algorithm that is optimized for lung cancer.”
They are looking for a suitable commercial assay platform for which they can build a standardised BARD1 Test – meaning that any lab using that platform could feasibly use their technology to test for ovarian or lung cancer.
“Our preference would be to licence this out to a large diagnostic partner, but many companies do need to take their test all the way through to commercialisation and get maybe $1 million in sales before a large company will look at them,” she said.
BARD1 backdoor listed onto the ASX in 2016 and also has a cancer vaccine in its pipeline.
Its last quarterly report showed a cash balance of $1.7 million, with $624 million in expected spending in the December quarter.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.