Study shows 81pc success rate for Orthocell Remplir nerve repair product

Orthocell delivers strong interim results from its Remplir Real World Evidence study. Pic via Getty.
- Interim results from Orthocell’s new Remplir study demonstrate treatment success rate of 81.1% following nerve repair procedures
- Results confirm Remplir as ideal for connecting severed nerves, protecting damaged nerves or capping nerves after amputation
- Study to support US Remplir sales roll out and as supporting evidence for EU and UK regulatory submission
Special Report: Perth-based regenerative medicine company Orthocell (ASX:OCC) has released positive interim results from its Remplir Real World Evidence (RWE) Study, demonstrating overall treatment success rate of 81.1% across a variety of nerve repair procedures.
Data on 49 patients aged 14 to 82 was included in the interim analysis. The patients underwent a total of 67 peripheral nerve procedures, mostly in the upper limb.
Most procedures (61.2%) were nerve reconstruction procedures for acute injury (including nerve transfer and nerve grafting) with 38.8% nerve decompression procedures using Remplir as a protective wrap in patients with chronic nerve injuries.
Safety data was available for all 67 procedures and no post-treatment complications or adverse reactions to the US Food and Drug Administration (FDA) approved Remplir were reported in any patient.
Performance data was available for 43 with the overall treatment success rate for all procedure types 81.1% (43 of 53 therapeutic targets).
Other key outcomes from the interim analysis include:
- Motor nerve reconstruction – 81.2% of muscles innervated by repaired nerves achieved functional recovery, on average 8.3 months post-surgery.
- Nerve decompression procedures – 89.5% resulted in significant improvement or complete relief of symptoms.
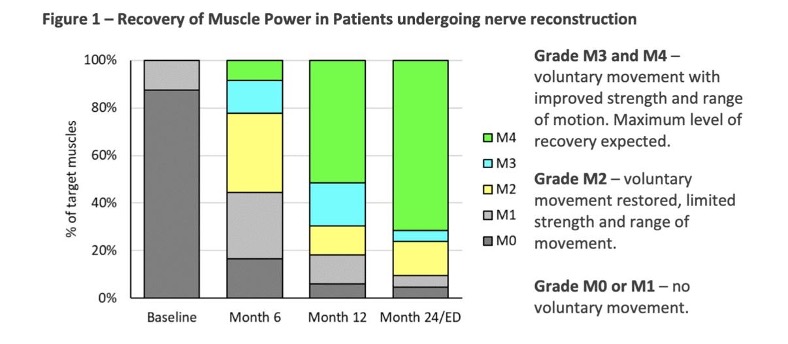
Source: Orthocell
About the study
The Remplir RWE study is an ongoing, multi-centre prospective and retrospective post-market clinical follow-up study in Australia.
The study is designed to collect safety and efficacy data on the use of Remplir, in a real-world setting.
It is being conducted in collaboration with Dr Alex O’Beirne at the Western Orthopaedic Clinic, associate professor Matthew Lawson-Smith at the Murdoch Orthopaedic Clinic, Jaslyn Cullen at Innervate Occupational Therapy, and Hand Works Occupational Therapy.
Eligible patients include those receiving Remplir during any peripheral nerve procedure, from acute traumatic injuries to chronic conditions causing pain, numbness or muscle paralysis.
Patients are eligible for inclusion in the study if they receive Remplir during any peripheral nerve procedure at a study site.
The study collects data on outcomes related to the goals of surgical treatment including restoration of hand function after cervical spinal cord injury and relief of symptoms caused by carpal tunnel syndrome for up to 24 months after treatment with Remplir.
Watch: Orthocell’s Remplir builds strong US market momentum
Consistent results with previous study
The results were consistent with results of previous study outcomes published in the Journal of Reconstructive Microsurgery Open.
The interim results highlight Orthocell’s ambition to make Remplir the gold standard in nerve repair surgery, delivering functional recovery for paralysed limbs and symptom relief for chronic nerve injuries.
“This performance is why more than 200 surgeons across more than 165 hospitals are now using Remplir and these numbers continue to grow,” CEO and managing director Paul Anderson said.
“This data is incredibly valuable from a commercial and regulatory perspective as it demonstrates the performance of Remplir across a heterogenous patient population in the real world, as distinct from a tightly controlled clinical trial environment.
“It is imperative that we continue to collect performance data to drive the rapid market adoption in the US and support the EU & UK regulatory applications.”
Study to support US rollout plus EU and UK regulatory submissions
The study will support Orthocell’s US sales rollout, ongoing medical education and planned EU and UK regulatory submissions expected in Q4 CY25.
The company said it was focused on expanding adoption in the US market, valued at ~US$1.6 billion, and preparing for launch in Canada.
With ~$27 million in cash and no debt, Orthocell said it was well-positioned to scale adoption, accelerate revenue growth in FY26 and ultimately target a total addressable market exceeding US$3.5 billion across selected jurisdictions.
This article was developed in collaboration with Orthocell, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.