Sienna is selling its pee testers in South Korea to sniff out bladder cancer

Pic: Godji10 / iStock / Getty Images Plus via Getty Images
Sienna Cancer Diagnostics has expanded into its sixth country, with a South Korean health distributor to sell its cancer detection test in the country.
Sienna (ASX:SDX) has developed an in-vitro test to detect the biomarker telomerase, which is prevalent in 85 percent of all tissue-based cancers. A biomarker is naturally occurring and identifies the presence of a disease.
The company told the market this morning that South Korean health distributor Mirax Corp would sell the product to Korean pathology labs, with the idea that it will be used in conjunction with urine cytology — a test for cancer in cells that are shed during urination — to better diagnose bladder cancer.
It’s the sixth country Sienna has signed a deal, following the US, Denmark, Sweden, Switzerland and China. It needs regulatory approval from South Korea before sales can begin, however.
- Subscribe to our daily newsletter
- Bookmark this link for small cap news
- Join our small cap Facebook group
- Follow us on Facebook or Twitter
Chief Matthew Hoskin said it was a “substantial” market opportunity, estimating there are more than 300,000 urine cytology tests performed in South Korea each year.
“We are delighted to have signed capable distributors in two key Asian markets in the past six months. This partnership is another step in our geographical expansion program and creates further opportunity in the fast-growing Asian IVD market,” he said.
Sang-Ju Bae, CEO of Mirax, said the hTERT test “could be a core growth driver for our business”.
“We have established ourselves as a quality provider in the Korean pathology market, with well-established networks and high-performing sales and technical support staff. Adding Sienna’s hTERT IVD test enhances our portfolio with a true value-adding diagnostic product that fits our capability and our strategic focus,” he said.
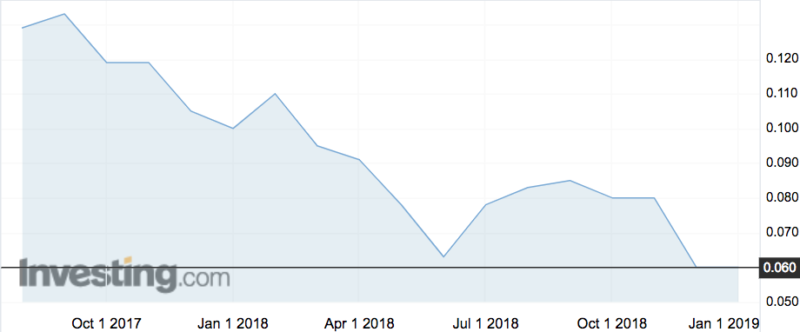
Sienna shares were flat on the news at 6c. The company’s shares were hit hard after its IPO, when greedy investors sold out for an early profit.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








