‘Remarkable outcome’: Paradigm achieves outstanding results in phase II osteoarthritis trial

Paradigm Pharmaceuticals achieves primary endpoints for Phase II osteoarthritis trial. Pic: Getty Images.
- Paradigm Pharmaceuticals phase II study for treatment of knee osteoarthritis reports successful outcome at day 356
- Clinical outcomes improvement observed for iPPS twice weekly compared to placebo control
- Company now intends to proceed with a Provisional Approval application to the TGA
Paradigm Pharmaceuticals has announced a breakthrough in the treatment of osteoarthritis with its phase II trial meeting its primary endpoints at day 56 and then demonstrating 12 months of pain reduction and functional improvement.
Good news for sufferers of osteoarthritis (OA) disease today with Paradigm Biopharmaceuticals (ASX:PAR) announcing positive results from its phase II clinical trial of injectable pentosan polysulfate sodium (iPPS).
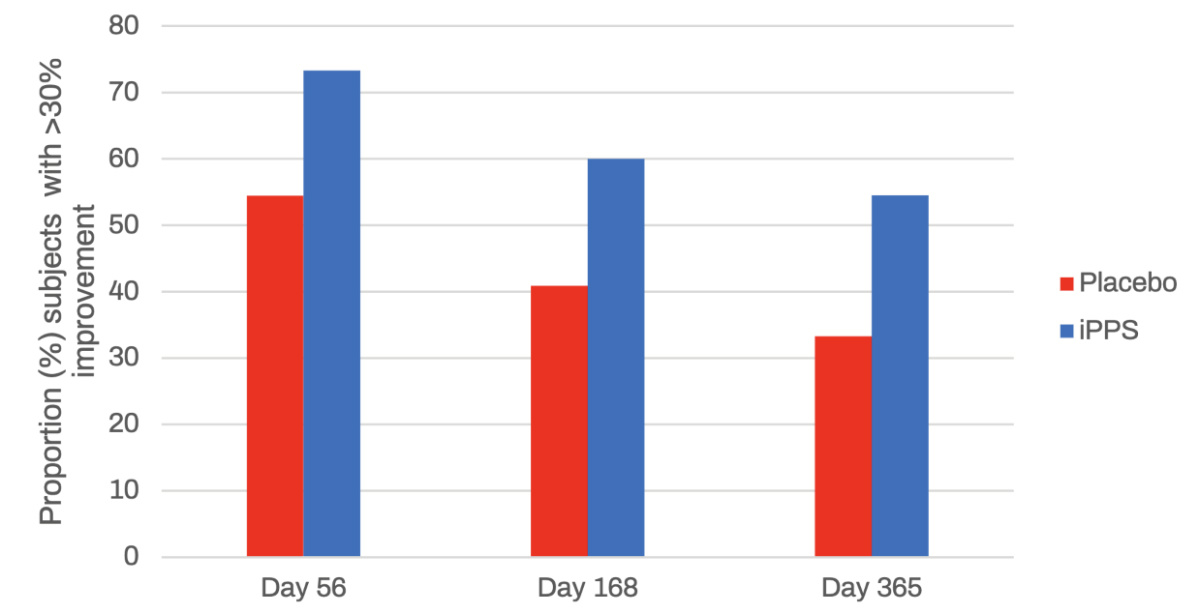
According to the study results, durable and significant responses in osteoarthritis scores for pain, function, stiffness and overall scores in participants were observed for iPPS administered twice weekly for six weeks compared to placebo control through to day 365.
However, the once weekly iPPS dose didn’t result in meaningful or sustainable improvement in clinical outcomes over placebo.
From a single 6-week iPPS treatment course, participants receiving twice weekly iPPS experienced persistent improvements in knee osteoarthritis pain compared to before treatment, out to the 12-month study endpoint.
The improved pain response for the twice weekly group peaked at day 56 and the response was sustained through to 12 months compared to the placebo group, which regressed towards baseline.
The phase II clinical trial was performed concurrently with the company’s ongoing phase III study.
 Source: PAR
Source: PAR
Furthermore, a 50% improvement in function was reported for 53% of participants receiving twice weekly iPPS at day 168, and for 55% of participants at day 365, compared to 22% and 28% in the placebo-treated group, respectively.
Patient Global Impression of Change
The Patient Global Impression of Change (PGIC) rating serves as a self-reported assessment that reflects a patient’s perception of the overall effectiveness of a treatment.
Participants assign a rating on a scale ranging from 1, indicating no change or a worsening condition, to 7, indicating a significant improvement that has had a substantial impact.
PGIC scores capture the patient’s viewpoint, which provides valuable insights when evaluating a treatment’s effectiveness beyond the more objective clinical measurements.
In Paradigm’s phase III clinical trial, designated as PARA_OA_002, an enhanced PGIC assessment serves as one of the endpoints.
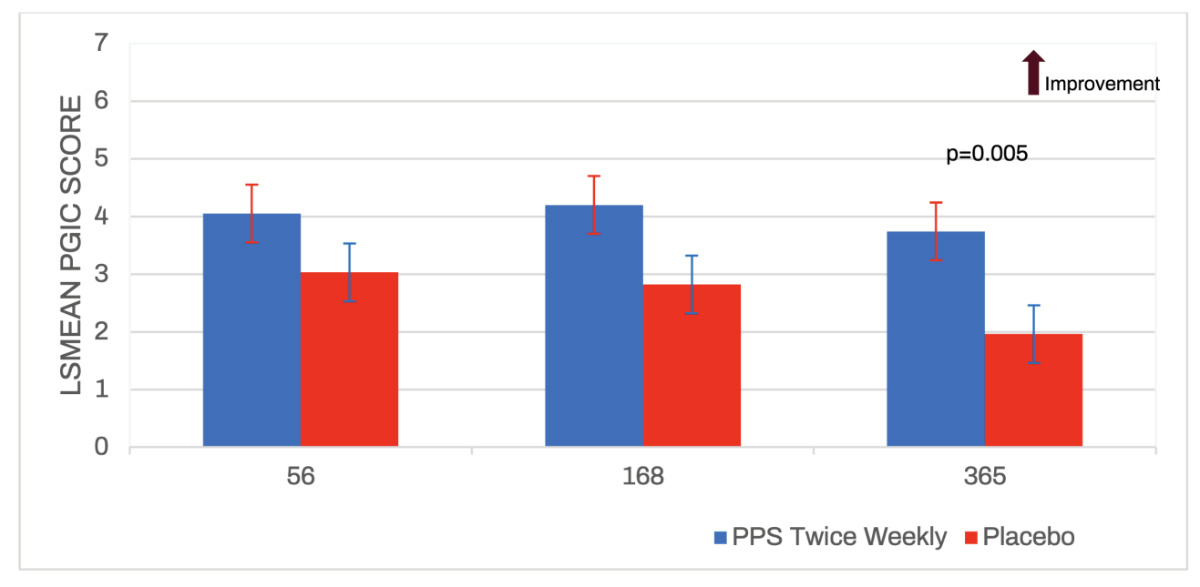
Average PGIC scores in the phase II study trended towards significance up to day 365 and then demonstrated highly statistically significant scores for the twice weekly iPPS group compared to placebo.
The average day 365 score was 3.74 for twice weekly iPPS versus 1.96 in the placebo group and indicated that participants receiving iPPS felt an overall improvement or stabilisation in their osteoarthritis disease progression.
Source: PAR
Rescue medication use lower
Rescue medication use provides an objective and quantifiable measure of a patient’s pain relief needs and is an important measure to determine how much additional pain relief participants require beyond the trial treatment.
Regulatory agencies, such as the US Food and Drug Administration (FDA), often consider rescue medication use when evaluating the efficacy of a new pain treatment.
Paracetamol was the allowable rescue medication during the study. In a positive outcome, the placebo group’s cumulative use of paracetamol remained more than 5 times higher at day 365 (28,947 mg) compared to the twice weekly iPPS group (5,147 mg).
PAR has previously reported positive results including attaining the primary endpoint at day 56, which was a change in one or more biomarkers of osteoarthritis progression in the knee joint, and secondary endpoints of improvement in osteoarthritis pain and function, and structural improvements in the osteoarthritic knee of reduced bone marrow lesions, reduced cartilage degradation, and reduced osteophytes (bone spurs) by MRI at day 168.
PAR to seek provisional approval with TGA
PAR says now that duration of effect of iPPS has been demonstrated out to 12-months, the company intends to proceed with a Provisional Approval application to the TGA.
The company is currently awaiting the full quantitative analysis of the six-month MRI data from the study and expects to report these findings shortly.
Overall results produced in the PARA_OA_008 phase II clinical trial are being compiled into a manuscript for peer review and publication.
PAR managing director Paul Rennie says to achieve clinically meaningful and significant results with iPPS at a dose of 2 mg/kg twice weekly compared to placebo at 12 months in only a small number of subjects per treatment arm shows clear strength in this treatment regimen over placebo.
“The 12-month durability of effect on OA pain and function following one 6-week course of treatment is truly an outstandingly positive trial outcome and separates iPPS from all currently available therapies for knee OA,” Rennie says.
“We have consistently received positive patient and prescribing doctor testimonials relating to iPPS use through the TGA Special Access Scheme,” he says.
“They indicate that a large proportion of patients receiving a dose of 2 mg/kg twice weekly are experiencing at least 12-months of reduced pain and improved function following the treatment course.
“The confirmation of our real-world evidence with this clinical data in a double-blinded placebo-controlled trial is a remarkable outcome for Paradigm. It also provides clarity for the appropriate dosing regimen for iPPS in treating knee osteoarthritis and support for the Company’s global partnering prospects moving forward.”
Treatment for common condition
Osteoarthritis is the most prevalent type of arthritis, often referred to as degenerative joint disease or wear and tear arthritis by some.
Osteoarthritis predominantly manifests in the hands, hips, knees, and feet with the protective cartilage within a joint deteriorating, leading to alterations in the underlying bone.
The global OA therapeutics market size was US$8.28 billion in 2022 and it is expected to reach around US$20.24 billion by 2032.
Medical director at Sportsmed Biologic in Melbourne and principal investigator of the trial Dr Philip Bloom says the mainstays in managing OA pain are simple analgesics like paracetamol or aspirin, along with more anti-inflammatory types of analgesics like ibuprofen or diclofenac (Voltaren), along with changes to diet and movement.
“We see osteoarthritis patients in the clinic when the simple analgesics have failed, the anti-inflammatories are failing, they’re not progressing with physiotherapy and their lifestyles are starting to be affected, so they come looking to us for help,” he says.
Bloom says OA sufferers can then try prescription-only pain medications, platelet rich plasma derivatives, joint lubricants like hyaluronic acid, or corticosteroid injections.
“There are pros and cons with each of them, but generally we find they’re only effective for a short period of time,” he says.
“After prescribing PPS to more than 300 patients via the TGA’s Special Access Scheme, I’ve seen a myriad of people seeing positive changes to their lifestyles and able to get back to things they previously may have been unable to do due to the pain and dysfunction caused by their disease.”
He says it also seems that most people receive meaningful pain relief out to about 18 months to two years, which is much longer than any currently available treatment.
“It would make sense to me to see PPS used as an earlier stage intervention to help maintain and preserve joint health and normal life activities, rather than only when the disease has significantly progressed,” he says.
This article was developed in collaboration with Paradigm Biopharmaceuticals, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








