Recce’s R327 shows promise against hospital superbug in preclinical studies

R327 shows promise in tackling a critical global health priority pathogen. Pic: Getty Images
- Recce’s R327 demonstrates significant antibacterial activity against multidrug-resistant pathogen Acinetobacter baumannii
- Considered one of the most challenging and antibiotic-resistant bacteria associated with hospital acquired infection
- Study builds on prior positive Murdoch Children’s Research Institute preclinical results, further demonstrating R327’s versatility
Special Report: One of the most challenging and antibiotic-resistant bacteria associated with hospital-acquired infections, carbapenem-resistant Acinetobacter baumannii (CRAB) (A. baumannii), has shown strong susceptibility to Recce Pharmaceuticals’ synthetic anti-infective candidate RECCE® 327 (R327) in preclinical studies.
The research, conducted by Murdoch Children’s Research Institute (MCRI), evaluated Recce Pharmaceuticals’ (ASX:RCE) R327 in validated mouse models of Hospital/Ventilator Acquired Pneumonia caused by CRAB – a critical global health priority pathogen.
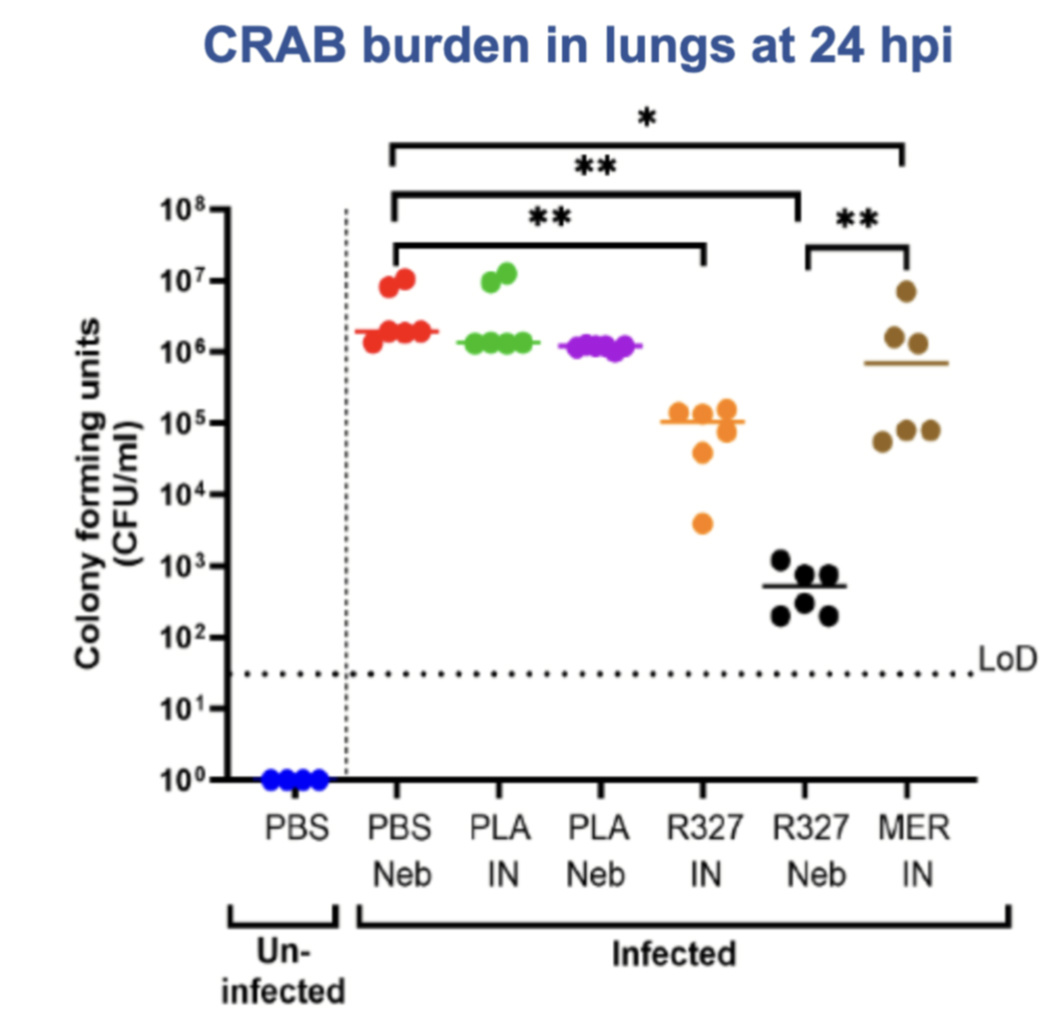
Forty female mice were assigned to seven treatment groups receiving either R327, placebo, saline, or meropenem – a last-resort treatment option, which can cause severe liver injury – by intranasal drops or nebulisation.
Unlike meropenem, which is challenging to nebulise due to solubility limitations – R327 can be effectively administered as a nebulised mist, allowing direct delivery to the lungs where infections occur.
At 24 hours post-infection, both intranasal and nebulised R327 treatments achieved strong bacterial clearance, with nebulised R327 delivering a 4-log reduction, corresponding to a >99.99% drop in lung bacterial burden.
Importantly, Recce said the nebulised R327 group achieved bacterial counts approaching the lower limit of detection (LoD), demonstrating potent local infection control.
Preliminary reductions in key pro-inflammatory markers were also observed in R327-treated groups, supporting its potential for controlling severe lung infections.
By comparison, meropenem reduced bacterial counts but because it is restricted to intranasal delivery, its practical use in clinical settings is limited.
Results further ‘validate the versatility of R327’
CEO James Graham said the results reinforce R327’s versatility as a potential inhaled therapy for serious multidrug-resistant lung infections, particularly in hospital and intensive care environments.
“These results further validate the versatility of R327, administered as an inhaled formulation, as a potential treatment for serious, drug-resistant lung infections,” he said.
“Unlike other antibiotics used against resistant pathogens, such as meropenem, which cannot be nebulised due to solubility limitations, R327 can be effectively delivered as a fine mist directly to the lung, precisely where the infection occurs.
“The potential to administer R327 via nebuliser or ventilator provides a significant real-world advantage in hospital settings, including intensive care and emergency environments where rapid, localised treatment is critical.”
Watch: Who’s Who with Recce Pharmaceuticals
Partnering with MCRI to support R327 development
MCRI is Australia’s largest child health research institute and is ranked among the top three globally for research quality and impact.
In 2023, Recce partnered with MCRI to establish the Anti-Infective Research (AIR) Unit, focused on evaluating R327 across a variety of infection models relevant to clinical care; including sepsis, wound, and respiratory infections.
Findings from these studies will support formulation optimisation, dose-response modelling, and regulatory submissions for R327’s future inhaled and topical development programs.
Lead Investigator at the AIR Unit Dr Sohinee Sarkar said the results contribute to a growing body of evidence highlighting R327’s potential to fight multidrug-resistant A. baumannii infections.
“Given the challenges in treating CRAB-related pneumonia, these findings are highly encouraging for future clinical translation,” Sarkar said.
This article was developed in collaboration with Recce Pharmaceuticals, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.