Radiopharmaceutical emerges as the hottest space in biotech; here’s what it means for Clarity Pharma

Radiopharmaceutical has become a hot space in biotech, and here’s where it puts ASX’s Clarity Pharma. Picture Getty
- Radiopharmaceutical is a hot space in biotech following recent deals
- Clarity is one of the only companies left with an advanced copper isotopes program
- Stockhead reached out to Clarity’s executive chairperson, Dr Alan Taylor
Radiopharmaceutical has emerged as arguably the hottest space in biotech right now.
Over the last six months, three global blockbuster deals have taken place in this space – AstraZeneca acquiring Fusion for US$2.4b, Eli Lilly buying out Point Biopharma for US$1.4b, and Bristol Myers Squibb’s purchase of Rayze Bio for US$4.1b.
The deals highlight the strong strategic interests in radiopharmaceutical assets, with these big pharma activities fuelling huge interest among investors.
What’s also interesting is that these acquisitions were made while the target’s lead programs were still in clinical trials, which is exactly where ASX-listed Clarity Pharmaceuticals (ASX:CU6) is at today.
The $760m market capped Clarity is a radiopharmaceutical-focused biotech which has developed a unique proprietary program involving the use of copper isotopes, in prostate cancer diagnosis and therapy.
“Radiopharmaceutical, in the global sense, is a hot market right now. It’s a very interesting space off the back of the antibody drug conjugate market which has already been hot,” said Clarity’s executive chairperson, Dr Alan Taylor.
Taylor adds that there is now an extremely limited number of clinically advanced radiopharmaceutical companies remaining which could provide Big Pharma with a platform entry point to radiopharmaceutical therapeutics.
“There’s no one left but us really in this exciting radiopharma space,” he said.
The company has recently completed an institutional cap raise of $110m, and has just launched a fully underwritten retail offer to raise up to a further $11 million at $2.55 per share.
“The total of $121 million significantly strengthens our balance sheet to over $150 million, and allows us to continue to progress all of our products through their respective clinical trials,” said Taylor.
This is the first capital raising since the company’s record $92 million IPO capital raising on the ASX in August 2021.
Why copper?
Since IPO, Clarity has stamped itself as a global leader in Targeted Copper Theranostics (TCTs), a disruptive platform in radiopharmaceutical that employs the “perfect pairing” of Copper-64 and Copper-67 for diagnosis and therapy (or theranostics), respectively.
“What we have and no one else has, is copper theranostics – a combination of copper diganostic and copper therapy,” explained Taylor.
“So we’re bringing to the market this perfect pairing of copper isotopes, Copper-64 and Copper-67.”
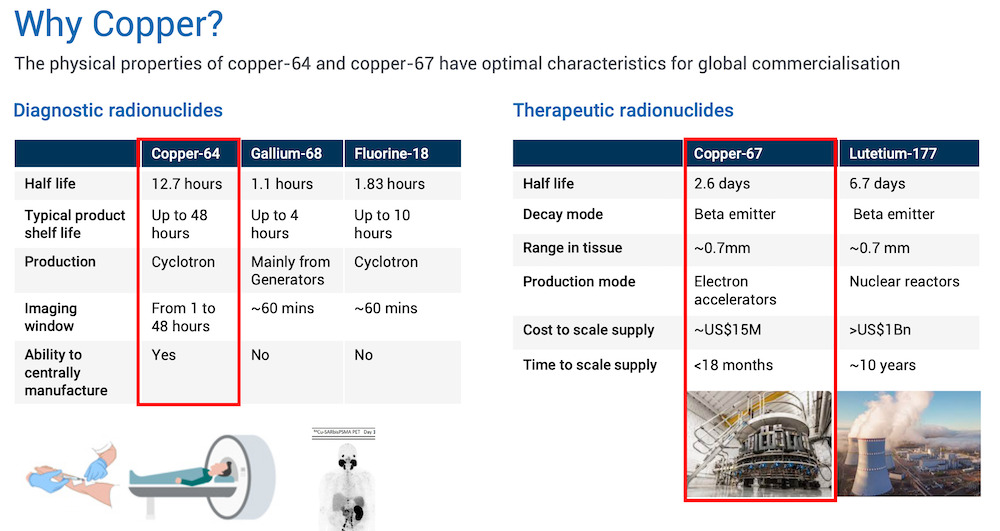
Taylor explains that Copper-64 (used for diagnosis), has a 12.7 hour half-life, and competes with currently used products like Gallium-68 and Fluorine-18, which only have a 1-2 hour half-life.
A short half-life can be problematic as a diagnosis agent for several reasons. First, a short half-life means there is only a limited time frame during which it can be detected in the body. And second, a short half-life may not allow enough time for the distribution of the agent in the body, resulting in poor imaging quality.
“Importantly for us however, we have this perfect pairing with an isotope called Copper-67, which is a therapeutic isotope,” said Taylor.
Copper-67 has a half-life of 2.6 days, compared to its competitor Lutetium-177, which has a half-life of 6.7 days.
“So Copper-67 is perfect for therapy, and it allows us to dose the patient a lot more on the same day,” said Taylor, adding that the shorter half-life results in shorter duration of radiation exposure to the patient and surrounding healthy tissues.
“That’s why it’s so exciting, because we basically own this copper thermal theranostic market,” he said.
Clarity also leverages its proprietary SAR Technology, a highly stable bifunctional cage (chelator), which enables a superior ability to retain copper isotopes within it and prevent their leakage into the body.
“We believe that at this stage, we have the best PSMA (prostate-specific membrane antigen) agents ever invented, and we have a market ready to accept that,” said Taylor.
Breaking down Clarity’s clinical program
Clarity’s program revolves around three key product areas, as shown below:
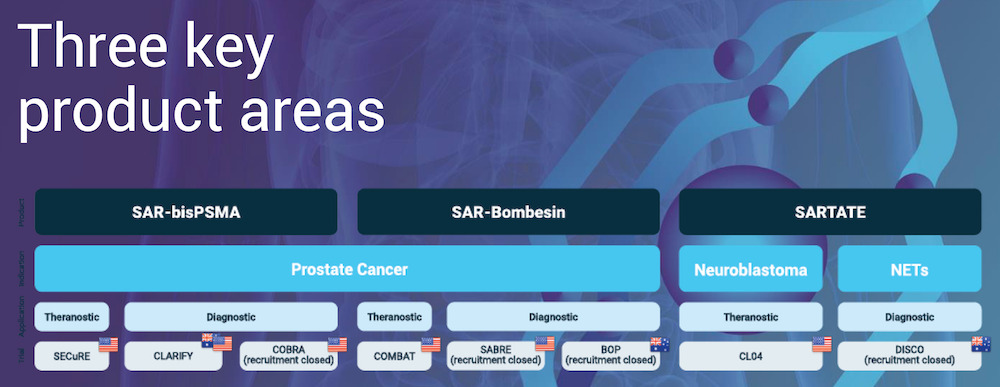
In a nutshell, the company’s various trials essentially use the same two products for diagnosis and therapy.
“The only thing we change is the copper isotope tag. So every time we invent a product, it’s actually just two products. One for diagnosis using Copper-64, and one for therapy using Copper-67,” explained Taylor.
Near term catalysts and focus at this stage are on the prostate cancer program, with SAR-bisPSMA and SAR-Bombesin being the two product areas.
In the theranostic SECuRE trial – which is investigating SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) patients – Clarity recently reported that the first patient in cohort 4 (first multi-dose cohort) has been treated with 67Cu-SAR-bisPSMA at 12GBq.
The SECuRE trial has so far shown remarkable results. Among the participants from cohorts 2 and 3, almost 80% showed reductions in PSA (prostate-specific antigen) levels of greater than 35% from a single dose of 67Cu-SAR-bisPSMA, and 44% showed reductions in PSA levels of greater than 80%.
“The PSA levels in those patients are basically gone, even in patient that had previously failed five to seven lines of therapy. This is amazing,” said Taylor.
“The overwhelming majority of interest in the market revolves around this incredible therapy data from SECuRE.”
Meanwhile, in the Phase 1/2 diagnostic COBRA trial, Clarity reported that Cu-SAR-bisPSMA was found to be safe and highly effective in detecting prostate cancer lesions in patients with biochemical recurrence (BCR).
For all lesions, regardless of their size, 64Cu-SAR-bisPSMA lesion uptake increased by more than 80%, and lesion contrast increased almost five times when comparing same-day to next-day imaging.
“The cornerstone of better therapy is better diagnosis, and we are incredibly excited about the substantial degree of improvement in detection of lesions with our bisPSMA product compared to SOC imaging,” said Taylor.
Other trials in the program are also moving forward rapidly; for example, the SABRE diagnostic trial has now imaged 50 patients in the US.
What’s ahead?
In all, Clarity has five open Investigational New Drug (IND) applications with the FDA, covering all six current clinical stage products that received clearance to proceed to clinical trials.
The company has also received approvals from the FDA for two Orphan Drug Designations (ODD), and two Rare Paediatric Disease Designations (RPDD).
Taylor says the next big catalyst will be the results from the SECuRE trial.
“When you look at big pharmas, they don’t necessarily love diagnostics but they love therapies. I don’t know of any big pharma that’s just focused on diagnostics.
“So, the value for Clarity is on the incredible therapeutic data we’ve got today,” he said.
“The market in this space is incredibly intense right now. There is a bunch of radiopharma companies that are being acquired without any real product.
“But our job is to focus on developing amazing products and differentiate ourselves from the rest of the market.
“We will focus on maintaining those barriers to entry, our strong intellectual property, because at the end of the day, we have to deliver these products to patients.
“That’s our primary job, and that’ll be our focus over the next period following the recent cap raise,” said Taylor.
Clarity Pharma share price today:
Another radipharmaceutical stock on the ASX
Radiopharm Theranostics (ASX:RAD)
Radiopharm is developing Nano-mAbs, a novel radiopharmaceutical platform made using genetic engineered camelid derived single domain antibodies (sdAb), labelled with a radioisotope of therapeutic radiation.
The product is paired with a diagnostic, using the same antibody vector but labelled with a lower radiation radioisotope for imaging.
In the latest update, RAD said that a study featuring DUNP19, (Radiopharm’s RAD 502) shows that it can halt tumour progression and prolong survival in various cancers.
The study, which was published on BioRxiv, demonstrated for the very first time how DUNP19 can be used in various cancers for the detection and targeting of Leucine-Rich Repeat Containing 15 (LRRC15)-expressing cancers.
LRRC15 is a cellular marker and new therapeutic target in solid tumours (breast, head and neck, lung, pancreatic), and in cancers that arise from connective tissue (osteosarcoma, glioblastoma, melanoma).
DUNP19 is currently under pre-clinical investigation at Radiopharm.
UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








