Race Oncology boosted by discovery of (E,E)-bisantrene’s anticancer mechanism

MYC inhibition is sometimes referred to as the ‘holy grail’ of cancer therapy. Pic: Getty Images.
- Race identifies primary mechanism of action for (E,E)-bisantrene (RCDS1), the active compound underpinning its lead candidate RC220 drug
- Targeting G4-DNA and RNA structures by RCDS1 reduces the activity of many important cancer genes, including the master gene regulator MYC
- Discovery presents major clinical and commercial implications and opportunities, including how to best use the drug as a new cancer treatment
Special Report: Race Oncology has achieved a breakthrough with its lead drug candidate RC220, identifying for the first time the primary mechanism of action (MOA) of the active pharmaceutical ingredient, (E,E)-bisantrene or RCDS1.
The discovery fundamentally reshapes how Race Oncology’s (ASX:RAC) cardioprotective anticancer drug RC220 can be applied in cancer treatment and provides significant advantages in clinical development, regulatory submissions and future partnering discussions.
Bisantrene was assumed to act in a similar way to widely used anthracyclines, such as doxorubicin – a chemotherapy class known for powerful anticancer activity but also for its severe cardiotoxic side effects.
Initially created by Lederle Laboratories in the 1970s and 1980s, bisantrene has the ability to provide protection of the heart caused by some chemotherapeutic drugs while also improving their anticancer activity.
While early studies suggested that bisantrene bound to DNA, its exact mechanism of action remained unclear, and clinical development of the compound was eventually abandoned.
From mystery to clarity
Race Oncology revived the molecule with the belief that its unique clinical profile, particularly its low-to-no cardiotoxicity, warranted further investigation.
Using modern molecular and cellular technologies unavailable in the 1980s, Race set out to answer the question that had eluded earlier researchers – how does bisantrene work?
Scientific studies conducted by Race and collaborators, have now revealed that RCDS1’s anticancer activity arises primarily through binding to and stabilising G-quadruplexes (G4s) – specialised three-dimensional structures formed in DNA and RNA.
In addition, stabilisation of G4-DNA and RNA structures shut down key growth enzymes (topoisomerase 2 and telomerase).
It alters RNA in a way that mimics blocking the fat mass and obesity-associated (FTO) protein, which is linked to cancer growth.
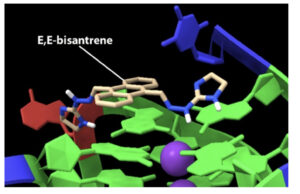
Model of RCDS1 (E,E-bisantrene) binding to the G4 DNA structure found within the promotor region of MYC. Source: Race Oncology
Importance of stabilising G4s to target cancer genes
G4 structures regulate the expression of many critical cancer genes, including the master cancer growth regulator MYC, one of the most important drivers of cancer growth, resistance and proliferation.
Race describes MYC as a master on-off switch for cell growth and division, activating the production of numerous proteins that drive the cell to divide.
“In normal cells, the job of MYC is to help control how quickly cells grow, copy their DNA, and divide,” the company said.
“It is tightly regulated, turning on only when the body needs new cells for growth, repair, or immune responses, and switches off when it is no longer needed.”
However, in cancer, MYC becomes overactive, and regulation is lost. As a result, MYC drives cancer cells to divide uncontrollably and alters their metabolism to support rapid tumour growth.
MYC is overexpressed or dysregulated in around 70% of cancers making it one of the most important targets for new anticancer treatments.
Importantly, Race has found that potent downregulation of the MYC gene occurs shortly after treatment RCDS1. The MYC-targeting has also been observed in other cancer cell types.
Could RCDS1 be the holy grail?
MYC inhibition is sometimes referred to as the ‘holy grail’ of cancer therapy – and that’s because of its central role in driving cancer cell proliferation.
Despite decades of research and investment by the pharmaceutical industry, the MYC gene has long been regarded as an ‘undruggable’ target with direct inhibitors repeatedly failing in development.
“In general, most successful drugs bind to specific pockets that exist in the target protein structure, much like how a key fits into a lock,” Race noted.
“The functional parts of the MYC protein are unstructured and lack drug binding pockets.
“This has hampered efforts to find potent MYC-inhibiting drugs despite a great deal of scientific effort.”
However, Race’s studies showing that RCDS1 indirectly suppresses MYC by stabilising the G4 structures that control its activity represents a breakthrough and a new way to inhibit one of the most important oncogenes in human cancer.
Race’s discovery positions RCDS1 as one of the most promising indirect MYC inhibitors in development.
Watch: RAC CEO Daniel Tillett to discuss the groundbreaking discovery of the primary mechanism of action of their lead drug.
Shift in clinical strategy following discovery
Understanding RCDS1’s true mechanism of action opens the door to a more targeted clinical strategy for Race with its drug candidate RC220.
Instead of broadly trialling across multiple cancers, Race can now prioritise cancer types that are most dependent on MYC and G4-regulated genes, significantly increasing the probability of clinical success.
For investors, the discovery of the primary mechanism of action for RCDS1 provides several critical advantages, including rational trial design.
By knowing how the primary active pharmaceutical works, Race can select the cancer sub-types and drug combinations most likely to respond, reducing costs and development timelines.
Race said regulators prefer the MOA of new drugs to be included in regulatory submissions since it improves patient safety by helping predict side effects, guide appropriate use and identify patients most likely to benefit.
Another advantage of knowing the MOA of RCDS1 is it can also enable development of pharmacodynamic biomarkers tests that are predictive of a patient’s response to treatment.
Historically, cancer drugs with an associated biomarker are more than twice as likely to achieve regulatory approval.
Understanding the MOA of a drug also increases the chances of partnering opportunities with Big Pharma companies expecting a clear mechanistic rationale before committing to late-stage development or commercial partnerships.
Race’s latest discovery de-risks RC220 and significantly strengthens its position in potential partnering discussions.
The latest discovery is further good news for the company. In September Race announced scientists had identified that bisantrene was composed of three distinct isomers – (E,E)-bisantrene, (E,Z)-bisantrene, and (Z,Z)-bisantrene – each with different biological properties and anticancer activity.
As a result, the company submitted a new ‘composition of matter’ patent application, which if granted would strengthen market exclusivity of its two bisantrene formulations – RC110 and RC220 – out to 2045.
Next steps after breakthrough
Race plans to build on this discovery with several key initiatives including:
- Further preclinical studies to explore G4-DNA and RNA binding effects on anticancer efficacy and resistance.
- Identification of lead cancer indications most suited to RCDS1 treatment.
- Assessment of synergistic drug combinations to enhance clinical outcomes.
- Development of biomarker assays to predict patient response.
- Peer-reviewed publication and conference presentations to share findings with the international oncology community.
‘Bisantrene continues to surprise’
Race Oncology CEO and managing director Dr Daniel Tillett said the discovery marked a major turning point for the company.
“The discovery that (E,E)-bisantrene acts primarily by binding to G4 DNA and RNA structures – and not like the chemotherapeutic doxorubicin – fundamentally changes our thinking on how best to use this drug in the clinic,” he said.
“Bisantrene continues to surprise, and we look forward to building on this mechanism of action discovery in our future clinical and commercial plans.”
This article was developed in collaboration with Race Oncology, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








