Punching the lights out: Best half year ever for MGC Pharma as it triples revenue, aims to convert clinical trials to sales

Pic: Getty Images
MGC Pharma has just delivered its best half ever, as it looks to complete a pipeline of clinical trials and convert them into commercial contracts.
The half saw MGC Pharma (ASX:MXC) achieve its strongest half-year result to date, with total sales of around $2.56 million.
The increase in sales revenue was largely underpinned by the strong demand for ArtemiC, the company’s non-cannabis COVID-19 supplement. ArtemiC’s sales totalled around $1.06 million, or 45% of all sales during the period.
During the half, MGC Pharma also sold around $1.25 million of its cannabinoid products.
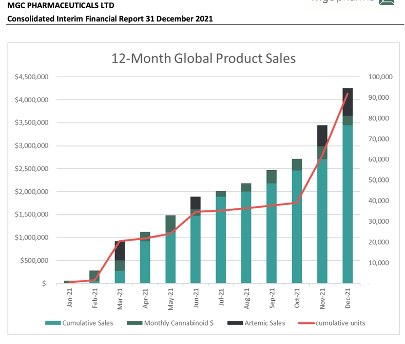
Overall, revenue for the half was $2.56m, which was an increase of 246% on pcp, while the bottom line showed a net loss of $8.05m, up 30% on pcp.
Milestones in the half
Key milestones achieved during the half include the completion of its Malta production facility, which is now due to be commissioned.
In addition, MGC Pharma has been granted a certificate of Free Trade in Europe for the company’s ArtemiC supplement, as well as getting an approval by the UK authorities for the import of CannEpil+.
“Further to this, we have made material strides in advancing Emergency Use Authorisation in multiple jurisdictions including the USA and India, as well as progressing stage II and III trials across the world,” said Roby Zomer, co-founder and CEO of MGC Pharma.
“As a company and management team, we are focused on gaining regulatory approval for our pharmaceutical products. Whilst this process is not always smooth, I remain confident in our products and our ability to achieve just this, which will result in better outcomes for patients globally,” he added.
Key US deal
In August last year, MXC announced the execution of a landmark supply and distribution agreement with US-based AMC Holdings Inc.
The terms included a minimum order of US$24 ($33) million over three years, with an initial first year order commitment of US$3 ($4.2) million, as well as a US$750,000 ($1.06 million) deposit which was received during the half-year.
The deal also stipulated that AMC will provide a National Clinical Trial Number to allow for MGC Pharma products to be imported into the US and used in clinical trials.
An initial order of 1,000 units of CimetrA has already been delivered to AMC during the period.
Those 1,000 units are expected to be used by research teams in the US, as the products undergo an Internal Review Board (IRB) examination at a number of institutions, including the University of South Florida, and the Holy Cross Hospital in Fort Lauderdale, Florida.
Acceptance by the IRB is the first step toward obtaining a National Clinical Trial Number.
The agreement with AMC is also a crucial step toward the expansion of MGC Pharma into the US market, which is a key part of the company’s overall growth strategy.
CimetrA approved for import in India
During the half, MGC Pharma was granted an approval to import samples of CimetrA into India by the Indian Central Drugs Standard Organisation.
The green light was given in order to facilitate final product testing required for the grant of Emergency Use Authorisation for the treatment of patients with COVID-19 in the country.
If the final testing phase is successful, the temporary import approval will be converted to a permanent approval.
Additionally, MGC Pharma also conducted an open label controlled observational trial for CimetrAT at the Mahatma Gandhi Mission’s Medical College and Hospital, as part of the Indian registration process.
European expansion
In September last year, the Medicines and Healthcare Products Regulatory Agency (MHRA) approved CannEpil+ for import into the UK through MGC Pharma’s clinical access partner, Elite PharmaCo.
This was the first time a cannabis-based treatment for epilepsy has been approved for import by UK authorities.
The treatment will be made available to ten patients on compassionate grounds for six months, with treatment data collected in an App provided by Alta Flora.
In continental Europe, MGC Pharma was granted a Certificate of Free Trade in Germany and the EU for the company’s proprietary nutraceutical non-cannabis food supplement, ArtemiC.
This follows the successful completion of its Phase II, multi centre clinical trial for ArtemiC, which demonstrated the enhanced recovery of patients with COVID-19 over the placebo control group. The German certificate is expected to accelerate entry of ArtemiC into both the EU as well as other markets.
Meanwhile, a clinical study for ArtemiC is underway with Swiss PharmaCan AG and Glow LifeTech, to determine the influence of ArtemiC Support in patients with Long COVID syndrome.
MXC has received an Ethics Committee approval, and the study will now enrol a total of 150 patients suffering from post-acute COVID syndrome.
Patients in the study will take ArtemiC Support for 6 weeks under supervision of their doctor, with their progress measured against a Post-COVID Functional Scale (PCFS), and a 10-point Likert scale, one, two, three and six weeks after treatment commences.
Malta Facility
Construction of MGC Pharma’s state-of-the-art production and manufacturing facility in Malta was completed on schedule in October, and within the parameters of the €3.1 ($4.8) million cash grant from the Maltese Government.
The production facility will have the capacity to produce over 20,000 units in liquid dose form per day, which is double that originally planned.
It is expected the Maltese facility will play a crucial part in MGC’s manufacturing pipeline, and to meet the expected near term demand from European markets.
The facility is currently going through the commissioning stage, whilst also completing the approval process to obtain a GMP licence.
Outlook
After successfully raising a £5.5 ($10.4) million share placement in October, MGC Pharma is ready to advance its CimetrA Emergency Use Authorisation applications, and testing procedures in five key countries in Central/Eastern Europe, and Central Asia.
The company will also seek to use the funds for the ongoing CimetrA dosing trials in the US and Russia, in order to comply with FDA prerequisites for approval, as well to support the CannEpil clinical trials in the US with its partner, AMC.
Looking ahead, MGC Pharma says it is focused on progressing those key clinical research programs, and continuing to expand its manufacturing capabilities in order to broaden its product range into new and existing key markets.
This article was developed in collaboration with MGC Pharma, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








