Prescient advances new therapies for hard-to-treat cancers

Prescient is tackling a rare form of non-Hodgkin lymphoma. Pic via Getty Images.
- Prescient is focused on developing new therapies for cancers of unmet need
- Lead compound PTX-100 is in a Phase II trial to treat rare blood cancer
- Strong US FDA support for PTX-100, including orphan drug and fast-track designations
Special Report: Clinical stage biotech Prescient Therapeutics is developing new therapies for cancers of unmet need, aggressive diseases that can devastate lives and for which treatment options remain limited.
Prescient Therapeutics (ASX: PTX) is currently focused on tackling cutaneous T-Cell lymphoma (CTCL), a rare form of non-Hodgkin lymphoma that primarily affects the skin, blood and internal organs, with its Phase II trial of lead compound PTX-100.
CTCL affects white blood cells known as T cells, which normally help regulate the immune system.
CEO James McDonnell said the disease began in the skin and was often misdiagnosed in its early stages, progressing into painful, disfiguring lesions and tumours.
Treatment options remain limited for patients whose disease has relapsed or become resistant to therapy.
Current treatments also have harsh side effects 98% of the time with 36% classified as serious.
First-in-class compound blocking cancer growth enzyme
PTX-100 is a first-in-class compound with the ability to block an important cancer growth enzyme geranylgeranyl transferase-1 (GGT-1).
McDonnell said PTX-100 disrupted critical signalling pathways that cancer cells relied on for growth and survival.
Specifically, it targeted the oncogenic Ras pathway, blocking the activity of Rho, Rac and Ral circuits.
The interference, he said, triggered a form of programmed cell death known as apoptosis, leading to the elimination of cancer cells.
PTX-100 is believed to be the only GGT-1 inhibitor in the world in clinical development.
Positive early trial results with PTX-100 now in Phase 2a trial
The compound demonstrated safety and early clinical activity in a previous phase I study along with a recent PK/PD basket study of haematological and solid malignancies.
In a phase 1b expansion cohort study in T cell lymphomas, PTX-100 showed
encouraging efficacy and safety.
Results saw a 45% overall response rate which McDonnell said meant they had a complete response or partial response to PTX-100 and a 64% clinical benefit.
PTX-100 showed only 4% serious adverse side effects with one case of diarrhea – only a fraction of the 30% target.
The CEO said the US Food and Drug Administration (FDA) was looking for drugs that address an unmet need for patients.
Safety was considered particularly important, not just because the patients were so fragile but also to enable the drug to be safely administered with other therapies like chemo.
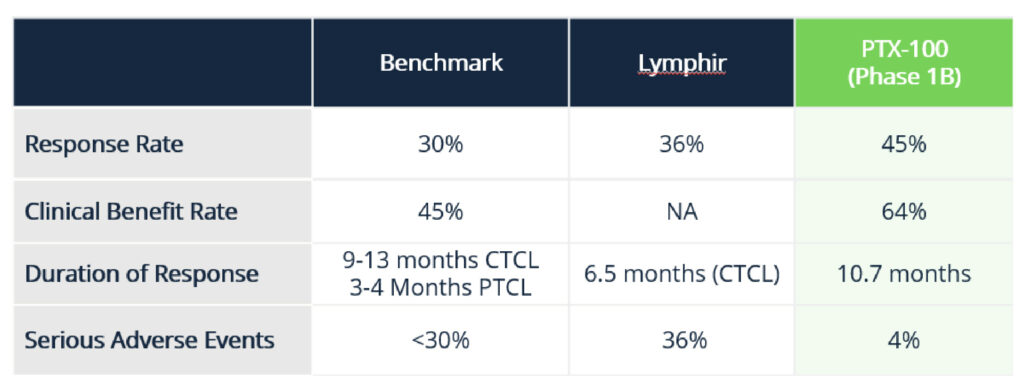
Source: Prescient Therapeutics
Phase II trial underway
PTX-100 is now in a Phase 2a clinical trial for patients with relapsed or refractory CTCL.
The trial will evaluate two dosage levels of PTX-100 in an open-label design in up to 40 patients with relapsed or refractory CTCL across multiple sites in Australia, the US and Europe.
If the Phase 2a trial shows an optimal dose and good efficacy, McDonnell said the company would work with the FDA to proceed to a phase 2b trial to evaluate safety and efficacy of PTX-100.
FDA support may fast-track trial process
The CEO is hopeful Prescient has a shot at becoming one of Australia’s biotech success stories with FDA designations, including orphan drug designation (ODD) for all T-cell lymphomas.
An ODD is aimed at encouraging treatment for rare disease and provides a range of benefits including seven years of market exclusivity upon approval in the US.
In December the FDA also cleared Prescient’s investigational new drug (IND) application, paving the way for human clinical trials of PTX-100 as a new drug to start in the US.
McDonnell is hopeful the phase 2b trial could be a registrational trial given PTX-100 has fast-track designation for treatment of adults with relapsed or refractory mycosis fungoides, the most common subtype of CTCL following the phase 1b results.
As the name implies fast-track designation implies faster process with the FDA through increased access.
If the FDA deems it appropriate for Phase 2b to be the registrational study, PTX-100 could skip years of additional trials and depending on results, go straight into market.
Prescient is also preparing to start a rolling new drug application (NDA) with the FDA, an option available to companies with fast-track designation that allows sections to be submitted as they’re ready, rather than waiting for a full submission.
The process aims to speed up review for therapies targeting serious or life-threatening conditions.
Targeting US$1.8 billion market for PTX-100 in treating TCL
With more than 27,000 new TCL cases every year in eight major centres, he said Prescient was aiming to get its first application for PTX-100 into market as fast as possible.
“PTX-100 has given us highly encouraging clinical data and we are now looking to take it to a US$1.8 billion market.”
The impact FDA support and designations could have for a company, he said, had seen biotech success stories like formerly Nasdaq-listed Mirati Therapeutics.
Mirati’s flagship drug transitioned from Phase I trials to commercialisation in as little as four years with strong FDA support and was acquired by Bristol Myers Squibb for US$4.8 billion – just 10 months after receiving accelerated approval.
“If Phase 2a trials show results anywhere near Phase 1b, then things will get very exciting,” McDonnell said.
“We could be in the market in very few years.
“The designations we have received from the FDA so far plus our Phase I results give us a lot of confidence.”
Larger opportunities for PTX-100 beyond CTCL
PTX-100’s Phase 2 trial is only focused on CTCL as it represents the most near-term, tangible opportunity showing significant results, but the company is also targeting other cancers.
One in five people will get cancer in their lifetime, while almost everyone has been touched by the disease in one way or another.
New global cancer cases are projected to increase by 77% from 20 million in 2022 to more than 35 million by 2050.
McDonnell said its first-in-class RAS pathway disruptor platform technology could apply to many cancers, including lung, bladder, ovarian and pancreatic cancers, to name a few.
There was a broader potential to improve the lives of millions of patients worldwide, he said.
“Our technology could apply to 22% of all cancers, so there is a significant opportunity for us to touch millions of lives.”
Advancing cell therapy technologies
On top of PTX-100, Prescient is also advancing its proprietary OmniCAR and CellPryme platforms which are relevant to CAR-T therapies. These are considered the next generation of cancer care.
A recent breakthrough immunotherapy trial using CAR-T cell therapy to wipe out solid tumours recently reported results that patients treated with the therapy lived on average approximately 40% longer.
McDonnell said Dr John Haanen, of the Netherlands Cancer Institute, thinks this is the new generation of treatment, opening significant opportunities to contribute to future groundbreaking therapies.
OmniCar is Prescient’s next generation CAR-T platform designed to give unprecedented control over cell therapies.
It has potential to allow CAR-T therapy cells to be more targeted, safer, more effective, cost-effective and of longer duration.
CellPryme is a complementary application to OmniCar split into two components.
CellPryme-M produces superior cells that are more potent and last longer, aiming to double tumour control. CellPryme-A acts as an adjuvant therapy, increasing the expansion of CAR-T cells and enhancing their ability to penetrate the tumour.
“We’ve got a drug in Phase 2 for a horrible disease of unmet need where we have high confidence from existing data and FDA support to take it into a US$1.8 billion market within a few years,” McDonnell added.
“That’s the near-term opportunity and 90% of our focus. But yes, the wider opportunity is compelling and with our other applications of PTX-100 plus our CAR-T platform we have a lot of opportunities.”
This article was developed in collaboration with Prescient Therapeutics, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
Related Topics

UNLOCK INSIGHTS
Discover the untold stories of emerging ASX stocks.
Daily news and expert analysis, it's free to subscribe.
By proceeding, you confirm you understand that we handle personal information in accordance with our Privacy Policy.








